- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Cranial nerve examination frequently appears in OSCEs. You’ll be expected to assess a subset of the twelve cranial nerves and identify abnormalities using your clinical skills. This cranial nerve examination OSCE guide provides a clear step-by-step approach to examining the cranial nerves, with an included video demonstration.
Gather equipment
Gather the appropriate equipment to perform cranial nerve examination:
- Pen torch
- Snellen chart
- Ishihara plates
- Ophthalmoscope and mydriatic eye drops (if necessary)
- Cotton wool
- Neuro-tip
- Tuning fork (512hz)
- Glass of water
Introduction
Wash your hands and don PPE if appropriate.
Introduce yourself to the patient including your name and role.
Confirm the patient’s name and date of birth.
Briefly explain what the examination will involve using patient-friendly language.
Gain consent to proceed with the examination.
Ask the patient to sit on a chair, approximately one arm’s length away.
Ask the patient if they have any pain before proceeding with the clinical examination.
General inspection
Perform a brief general inspection of the patient, looking for clinical signs suggestive of underlying pathology:
- Speech abnormalities: may indicate glossopharyngeal or vagus nerve pathology.
- Facial asymmetry: suggestive of facial nerve palsy.
- Eyelid abnormalities: ptosis may indicate oculomotor nerve pathology.
- Pupillary abnormalities: mydriasis occurs in oculomotor nerve palsy.
- Strabismus: may indicate oculomotor, trochlear or abducens nerve palsy.
- Limbs: pay attention to the patient’s arms and legs as they enter the room and take a seat noting any abnormalities (e.g. spasticity, weakness, wasting, tremor, fasciculation) which may suggest the presence of a neurological syndrome).
Look for objects or equipment on or around the patient that may provide useful insights into their medical history and current clinical status:
- Walking aids: gait issues are associated with a wide range of neurological pathology including Parkinson’s disease, stroke, cerebellar disease and myasthenia gravis.
- Hearing aids: often worn by patients with vestibulocochlear nerve issues (e.g. Ménière’s disease).
- Visual aids: the use of visual prisms or occluders may indicate underlying strabismus.
- Prescriptions: prescribing charts or personal prescriptions can provide useful information about the patient’s recent medications.
Olfactory nerve (CN I)
The olfactory nerve (CN I) transmits sensory information about odours to the central nervous system where they are perceived as smell (olfaction). There is no motor component to the olfactory nerve.
Ask the patient if they have noticed any recent changes to their sense of smell.
Olfaction can be tested more formally using different odours (e.g. lemon, peppermint), or more formally using the University of Pennsylvania smell identification test. However, this is unlikely to be required in an OSCE.
Causes of anosmia
There are many potential causes of anosmia including:
- Mucous blockage of the nose: preventing odours from reaching the olfactory nerve receptors.
- Head trauma: can result in shearing of the olfactory nerve fibres leading to anosmia.
- Genetics: some individuals have congenital anosmia.
- Parkinson’s disease: anosmia is an early feature of Parkinson’s disease.
- COVID-19: transient anosmia is a common feature of COVID-19.
Optic nerve (CN II)
The optic nerve (CN II) transmits sensory visual information from the retina to the brain. There is no motor component to the optic nerve.
Inspect the pupils
The pupil is the hole in the centre of the iris that allows light to enter the eye and reach the retina.
Assess pupil size:
- Normal pupil size varies between individuals and depends on lighting conditions (i.e. smaller in bright light, larger in the dark).
- Pupils are usually smaller in infancy and larger in adolescence.
Assess pupil shape:
- Pupils should be round, abnormal shapes can be congenital or due to pathology (e.g. posterior synechiae associated with uveitis).
- Peaked pupils in the context of trauma are suggestive of globe injury.
Assess pupil symmetry:
- Note any asymmetry in pupil size between the pupils (anisocoria). This may be longstanding and non-pathological or relate to actual pathology. If the pupil is more pronounced in bright light this would suggest that the larger pupil is the abnormal pupil, if more pronounced in dark this would suggest the smaller pupil is abnormal.
- Examples of asymmetry include a large pupil in oculomotor nerve palsy and a small and reactive pupil in Horner’s syndrome.
Visual acuity
Assessment of visual acuity (distance)
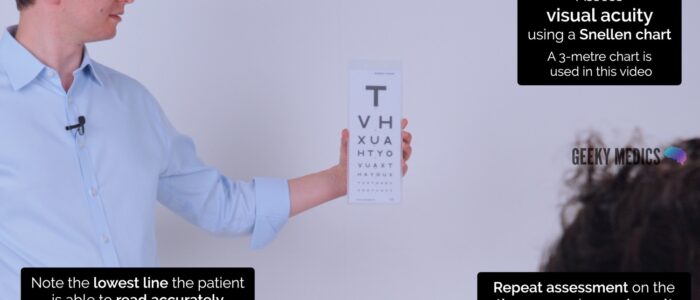
Begin by assessing the patient’s visual acuity using a Snellen chart. If the patient normally uses distance glasses, ensure these are worn for the assessment.
1. Stand the patient at 6 metres from the Snellen chart.
2. Ask the patient to cover one eye and read the lowest line they are able to.
3. Record the lowest line the patient was able to read (e.g. 6/6 (metric) which is equivalent to 20/20 (imperial)).
4. You can have the patient read through a pinhole to see if this improves vision (if vision is improved with a pinhole, it suggests there is a refractive component to the patient’s poor vision).
5. Repeat the above steps with the other eye.
Recording visual acuity
Visual acuity is recorded as chart distance (numerator) over the number of the lowest line read (denominator).
If the patient reads the 6/6 line but gets 2 letters incorrect, you would record as 6/6 (-2).
If the patient gets more than 2 letters wrong, then the previous line should be recorded as their acuity.
When recording the vision it should state whether this vision was unaided (UA), with glasses or with pinhole (PH).
Further steps for patients with poor vision
If the patient is unable to read the top line of the Snellen chart at 6 metres (even with pinhole) move through the following steps as necessary:
1. Reduce the distance to 3 metres from the Snellen chart (the acuity would then be recorded as 3/denominator).
2. Reduce the distance to 1 metre from the Snellen chart (1/denominator).
3. Assess if they can count the number of fingers you’re holding up (recorded as “Counting Fingers” or “CF”).
4. Assess if they can see gross hand movements (recorded as “Hand Movements” or “HM”).
5. Assess if they can detect light from a pen torch shone into each eye (“Perception of Light”/”PL” or “No Perception of Light”/”NPL”).
Causes of decreased visual acuity
Decreased visual acuity has many potential causes including:
- Refractive errors
- Amblyopia
- Ocular media opacities such as cataract or corneal scarring
- Retinal diseases such as age-related macular degeneration
- Optic nerve (CN II) pathology such as optic neuritis
- Lesions higher in the visual pathways
Optic nerve (CN II) pathology usually causes a decrease in acuity in the affected eye. In comparison, papilloedema (optic disc swelling from raised intracranial pressure), does not usually affect visual acuity until it is at a late stage.
Pupillary reflexes
With the patient seated, dim the lights in the assessment room to allow you to assess pupillary reflexes effectively.
Direct pupillary reflex
Assess the direct pupillary reflex:
- Shine the light from your pen torch into the patient’s pupil and observe for pupillary restriction in the ipsilateral eye.
- A normal direct pupillary reflex involves constriction of the pupil that the light is being shone into.
Consensual pupillary reflex
Assess the consensual pupillary reflex:
- Once again shine the light from your pen torch into the same pupil, but this time observe for pupillary restriction in the contralateral eye.
- A normal consensual pupillary reflex involves the contralateral pupil constricting as a response to light entering the eye being tested.
Swinging light test
Move the pen torch rapidly between the two pupils to check for a relative afferent pupillary defect (see more details below).
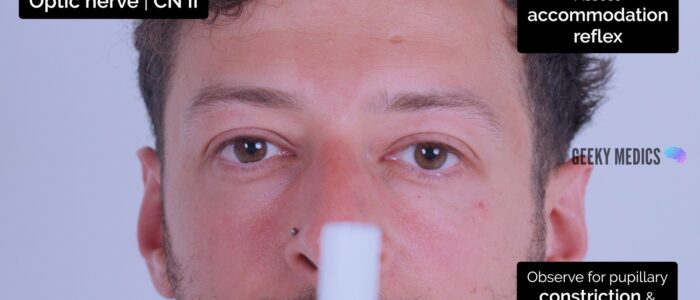
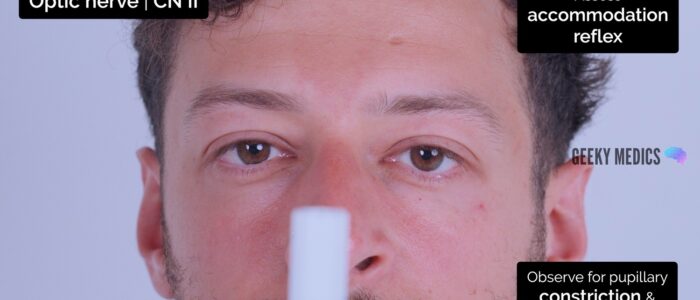
Accommodation reflex
1. Ask the patient to focus on a distant object (clock on the wall/light switch).
2. Place your finger approximately 20-30cm in front of their eyes (alternatively, use the patient’s own thumb).
3. Ask the patient to switch from looking at the distant object to the nearby finger/thumb.
4. Observe the pupils, you should see constriction and convergence bilaterally.
Pupillary light reflex
Each afferent limb of the pupillary reflex has two efferent limbs, one ipsilateral and one contralateral.
The afferent limb functions as follows:
- Sensory input (e.g. light being shone into the eye) is transmitted from the retina, along the optic nerve to the ipsilateral pretectal nucleus in the midbrain.
The two efferent limbs function as follows:
- Motor output is transmitted from the pretectal nucleus to the Edinger-Westphal nuclei on both sides of the brain (ipsilateral and contralateral).
- Each Edinger-Westphal nucleus gives rise to efferent nerve fibres which travel in the oculomotor nerve to innervate the ciliary sphincter and enable pupillary constriction.
Normal pupillary light reflexes rely on the afferent and efferent pathways of the reflex arc being intact and therefore provide an indirect way of assessing their function:
- The direct pupillary reflex assesses the ipsilateral afferent limb and the ipsilateral efferent limb of the pathway.
- The consensual pupillary reflex assesses the contralateral efferent limb of the pathway.
- The swinging light test is used to detect relative afferent limb defects.
Abnormal pupillary responses
- Relative afferent pupillary defect (Marcus-Gunn pupil): normally light shone into either eye should constrict both pupils equally (due to the dual efferent pathways described above). When the afferent limb in one of the optic nerves is damaged, partially or completely, both pupils will constrict less when light is shone into the affected eye compared to the healthy eye. The pupils, therefore, appear to relatively dilate when swinging the torch from the healthy to the affected eye. This is termed a relative…. afferent… pupillary defect. This can be due to significant retinal damage in the affected eye secondary to central retinal artery or vein occlusion and large retinal detachment; or due to significant optic neuropathy such as optic neuritis, unilateral advanced glaucoma and compression secondary to tumour or abscess.
- Unilateral efferent defect: commonly caused by extrinsic compression of the oculomotor nerve, resulting in the loss of the efferent limb of the ipsilateral pupillary reflexes. As a result, the ipsilateral pupil is dilated and non-responsive to light entering either eye (due to loss of ciliary sphincter function). The consensual light reflex in the unaffected eye would still be present as the afferent pathway (i.e. optic nerve) of the affected eye and the efferent pathway (i.e. oculomotor nerve) of the unaffected eye remain intact.
Colour vision assessment
Colour vision can be assessed using Ishihara plates, each of which contains a coloured circle of dots. Within the pattern of each circle are dots which form a number or shape that is clearly visible to those with normal colour vision and difficult or impossible to see for those with a red-green colour vision defect.
How to use Ishihara plates
If the patient normally wears glasses for reading, ensure these are worn for the assessment.
1. Ask the patient to cover one of their eyes.
2. Then ask the patient to read the numbers on the Ishihara plates. The first page is usually the ‘test plate’ which does not test colour vision and instead assesses contrast sensitivity. If the patient is unable to read the test plate, you should document this.
3. If the patient is able to read the test plate, you should move through all of the Ishihara plates, asking the patient to identify the number on each. Once the test is complete, you should document the number of plates the patient identified correctly, including the test plate (e.g. 13/13).
4. Repeat the assessment on the other eye.
Colour vision deficiencies
Colour vision deficiencies can be congenital or acquired. Some causes of acquired colour vision deficiency include:
- Optic neuritis: results in a reduction of colour vision (typically red).
- Vitamin A deficiency
- Chronic solvent exposure
Visual neglect/inattention
Visual neglect (also known as visual inattention) is a condition in which an individual develops a deficit in their awareness of one side (hemispace) of their visual field. This typically occurs in the context of parietal lobe injury after stroke, which results in an inability to perceive or process stimuli on one side of the body. It should be noted that visual neglect is not caused by optic nerve pathology, however, it is useful in the context of a cranial nerve examination to differentiate between pathology affecting the optic nerve (true visual field loss) and pathology affecting the cerebral hemisphere (inattention).
Assessment
To assess for visual neglect:
1. Position yourself sitting opposite the patient approximately 1 metre away.
2. Ask the patient to remain focused on a fixed point on your face (e.g. nose) and to state if they see your left, right or both hands moving.
3. Hold your hands out laterally, with each occupying one side of the patient’s visual field (i.e. left and right).
4. Take turns wiggling a finger on each hand to see if the patient is able to correctly identify which finger has moved.
5. Finally wiggle a finger on both hands simultaneously to see if the patient is able to correctly identify this.
Interpretation
Visual inattention is present when there is neglect of one hemispatial field (i.e. left or right hemispace). Neglect of the left hemispacial field is much more common.
Visual extinction is present when the patient can’t identify one of the moving fingers when a finger on both hands is wiggling simultaneously. They can, however, identify each of them when they’re wiggled individually. Again, during simultaneous bilateral wiggling, people will usually fail to see the finger wiggling on the left side.
Visual fields
This method of assessment relies on comparing the patient’s visual field with your own and therefore for it to work:
- you need to position yourself, the patient and the target correctly (see details below).
- you need to have normal visual fields and a normal-sized blindspot.
Visual field assessment
1. Sit directly opposite the patient, at a distance of around 1 metre.
2. Ask the patient to cover one eye with their hand.
3. If the patient covers their right eye, you should cover your left eye (mirroring the patient).
4. Ask the patient to focus on part of your face (e.g. nose) and not move their head or eyes during the assessment. You should do the same and focus your gaze on the patient’s face.
5. As a screen for central visual field loss or distortion, ask the patient if any part of your face is missing or distorted. A formal assessment can be completed with an Amsler chart.
6. Position the hatpin (or another visual target such as your finger) at an equal distance between you and the patient (this is essential for the assessment to work).
7. Assess the patient’s peripheral visual field by comparing to your own and using the target. Start from the periphery and slowly move the target towards the centre, asking the patient to report when they first see it. If you are able to see the target but the patient cannot, this would suggest the patient has a reduced visual field.
8. Repeat this process for each visual field quadrant, then repeat the entire process for the other eye.
9. Document your findings.
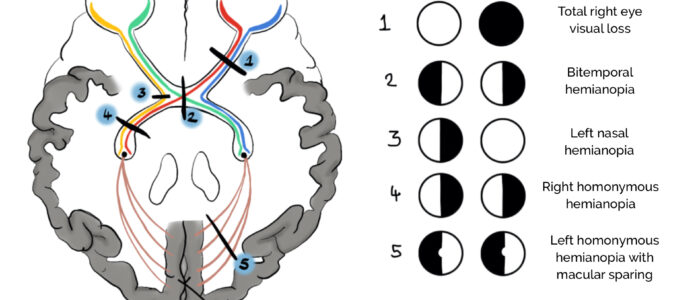
Types of visual field defects
- Bitemporal hemianopia: loss of the temporal visual field in both eyes resulting in central tunnel vision. Bitemporal hemianopia typically occurs as a result of optic chiasm compression by a tumour (e.g. pituitary adenoma, craniopharyngioma).
- Homonymous field defects: affect the same side of the visual field in each eye and are commonly attributed to stroke, tumour, abscess (i.e. pathology affecting visual pathways posterior to the optic chiasm). These are deemed hemianopias if half the vision is affected and quadrantanopias if a quarter of the vision is affected.
- Scotoma: an area of absent or reduced vision surrounded by areas of normal vision. There is a wide range of possible aetiologies including demyelinating disease (e.g. multiple sclerosis) and diabetic maculopathy.
- Monocular vision loss: total loss of vision in one eye secondary to optic nerve pathology (e.g. anterior ischaemic optic neuropathy) or ocular diseases (e.g. central retinal artery occlusion, total retinal detachment).
Blind spot
A physiological blind spot exists in all healthy individuals as a result of the lack of photoreceptor cells in the area where the optic nerve passes through the optic disc. In day to day life, the brain does an excellent job of reducing our awareness of the blind spot by using information from other areas of the retina and the other eye to mask the defect.
Blind spot assessment
1. Sit directly opposite the patient, at a distance of around 1 metre.
2. Ask the patient to cover one eye with their hand.
3. If the patient covers their right eye, you should cover your left eye (mirroring the patient).
4. Ask the patient to focus on part of your face (e.g. nose) and not move their head or eyes during the assessment. You should do the same and focus your gaze on the patient’s face.
5. Using a red hatpin (or alternatively, a cotton bud stained with fluorescein/pen with a red base) start by identifying and assessing the patient’s blind spot in comparison to the size of your own. The red hatpin needs to be positioned at an equal distance between you and the patient for this to work.
6. Ask the patient to say when the red part of the hatpin disappears, whilst continuing to focus on the same point on your face.
7. With the red hatpin positioned equidistant between you and the patient, slowly move it laterally until the patient reports the disappearance of the top of the hatpin. The blind spot is normally found just temporal to central vision at eye level. The disappearance of the hatpin should occur at a similar point for you and the patient.
8. After the hatpin has disappeared for the patient, continue to move it laterally and ask the patient to let you know when they can see it again. The point at which the patient reports the hatpin re-appearing should be similar to the point at which it re-appears for you (presuming the patient and you have a normal blind spot).
9. You can further assess the superior and inferior borders of the blind spot using the same process.
Fundoscopy
In the context of a cranial nerve examination, fundoscopy is performed to assess the optic disc for signs of pathology (e.g. papilloedema). You should offer to perform fundoscopy in your OSCE, however, it may not be required. See our dedicated fundoscopy guide for more details.
Oculomotor (CN III), trochlear (CN IV) and abducens (CN VI) nerves
The oculomotor (CN III), trochlear (CN IV) and abducens (CN VI) nerves transmit motor information to the extraocular muscles to control eye movement and eyelid function. The oculomotor nerve also carries parasympathetic fibres responsible for pupillary constriction.
Eyelids
Inspect the eyelids for evidence of ptosis which can be associated with:
- Oculomotor nerve pathology
- Horner’s syndrome
- Neuromuscular pathology (e.g. myasthenia gravis)
Eye movements
Briefly assess for abnormalities of eye movements which may be caused by underlying cranial nerve palsy (e.g. oculomotor, trochlear, abducens, vestibular nerve pathology).
1. Hold your finger (or a pin) approximately 30cm in front of the patient’s eyes and ask them to focus on it. Look at the eyes in the primary position for any deviation or abnormal movements.
2. Ask the patient to keep their head still whilst following your finger with their eyes. Ask them to let you know if they experience any double vision or pain.
3. Move your finger through the various axes of eye movement in a ‘H’ pattern.
4. Observe for any restriction of eye movement and note any nystagmus (which may suggest vestibular nerve pathology or stroke).
Actions of the extraocular muscles
- Superior rectus: primary action is elevation, secondary actions include adduction and medial rotation of the eyeball.
- Inferior rectus: primary action is depression, secondary actions include adduction and lateral rotation of the eyeball.
- Medial rectus: adduction of the eyeball.
- Lateral rectus: abduction of the eyeball.
- Superior oblique: depresses, abducts and medially rotates the eyeball.
- Inferior oblique: elevates, abducts and laterally rotates the eyeball.
Oculomotor, trochlear and abducens nerve palsy
Damage to any of the three cranial nerves innervating the extraocular muscles can result in paralysis of the corresponding muscles.
Oculomotor nerve palsy (CN III)
The oculomotor nerve supplies all extraocular muscles except the superior oblique (CNIV) and the lateral rectus (CNVI). Oculomotor palsy (a.k.a. ‘third nerve palsy’), therefore, results in the unopposed action of both the lateral rectus and superior oblique muscles, which pull the eye inferolaterally. As a result, patients typically present with a ‘down and out’ appearance of the affected eye.
Oculomotor nerve palsy can also cause ptosis (due to a loss of innervation to levator palpebrae superioris) as well as mydriasis due to the loss of parasympathetic fibres responsible for innervating to the sphincter pupillae muscle.
Trochlear nerve palsy (CN IV)
The only muscle the trochlear nerve innervates is the superior oblique muscle. As a result, trochlear nerve palsy (‘fourth nerve palsy’) typically results in vertical diplopia when looking inferiorly, due to loss of the superior oblique’s action of pulling the eye downwards. Patients often try to compensate for this by tilting their head forwards and tucking their chin in, which minimises vertical diplopia. Trochlear nerve palsy also causes torsional diplopia (as the superior oblique muscle assists with intorsion of the eye as the head tilts). To compensate for this, patients with trochlear nerve palsy tilt their head to the opposite side, in order to fuse the two images together.
Abducens nerve palsy (CN VI)
The abducens nerve (CN VI) innervates the lateral rectus muscle. Abducens nerve palsy (‘sixth nerve palsy’) results in unopposed adduction of the eye (by the medial rectus muscle), resulting in a convergent squint. Patients typically present with horizontal diplopia which is worsened when they attempt to look towards the affected side.
Assessment of strabismus
Strabismus is a condition in which the eyes do not properly align with each other when looking at an object. Pathology affecting the oculomotor, trochlear or abducens nerves can cause strabismus.
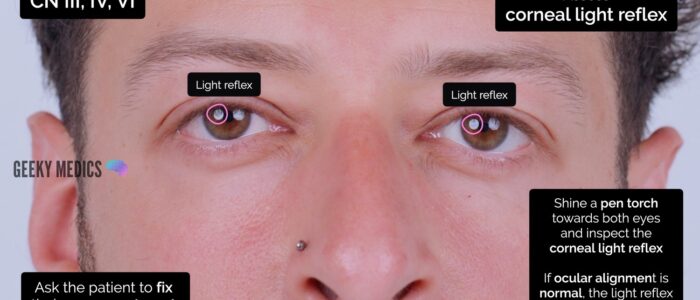
Light reflex test (a.k.a. corneal light reflex test or Hirschberg test)
1. Ask the patient to focus on a target approximately half a metre away whilst you shine a pen torch towards both eyes.
2. Inspect the corneal light reflex on each eye:
- If the ocular alignment is normal, the light reflex will be positioned centrally and symmetrically in each pupil.
- Deflection of the corneal light reflex in one eye suggests a misalignment.
Cover test
The cover test is used to determine if a heterotropia (i.e. manifest strabismus) is present.
1. Ask the patient to fixate on a target (e.g. light switch).
2. Occlude one of the patient’s eyes and observe the contralateral eye for a shift in fixation:
- If there is no shift in fixation in the contralateral eye, while covering either eye, the patient is orthotropic (i.e. normal alignment).
- If there is a shift in fixation in the contralateral eye, while covering the other eye, the patient has a heterotropia.
3. Repeat the cover test on the other eye.
The direction of the shift in fixation determines the type of tropia; the table below describes the appropriate interpretation.
Interpretation of the cover test
| Direction of eye at rest | The direction of shift in fixation of the unoccluded eye when the opposite eye is occluded | Type of tropia present |
| Temporally (i.e. laterally or outwards) | Nasally (i.e. medially or inwards) | Exotropia |
| Nasally (i.e. medially or inwards) | Temporally (i.e. laterally or outwards) | Esotropia |
| Superiorly (i.e. upwards) | Inferiorly (i.e. downwards) | Hypertropia |
| Inferiorly (i.e. downwards) | Superiorly (i.e. upwards) | Hypotropia |
Trigeminal nerve (CN V)
The trigeminal nerve (CN V) transmits both sensory information about facial sensation and motor information to the muscles of mastication.
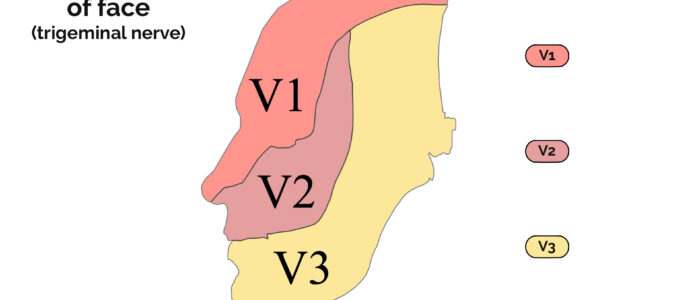
The trigeminal nerve has three sub-divisions, each of which has its own broad set of functions (not all are covered below):
- Ophthalmic (V1): carries sensory information from the scalp and forehead, nose, upper eyelid as well as the conjunctiva and cornea of the eye.
- Maxillary (V2): carries sensory information from the lower eyelid, cheek, nares, upper lip, upper teeth and gums.
- Mandibular (V3): carries sensory information from the chin, jaw, lower lip, mouth, lower teeth and gums. Also carries motor information to the muscles of mastication (masseter, temporal muscle and the medial/lateral pterygoids) as well as the tensor tympani, tensor veli palatini, mylohyoid and digastric muscles.
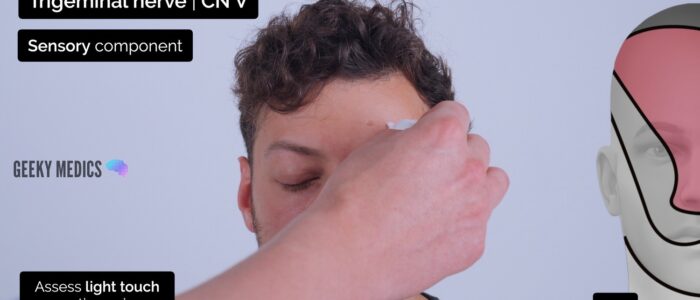
Sensory assessment
First, explain the modalities of sensation you are going to assess (e.g. light touch/pinprick) to the patient by demonstrating on their sternum. This provides them with a reference of what the sensation should feel like (assuming they have no sensory deficits in the region overlying the sternum).
Ask the patient to close their eyes and say ‘yes’ each time they feel you touch their face.
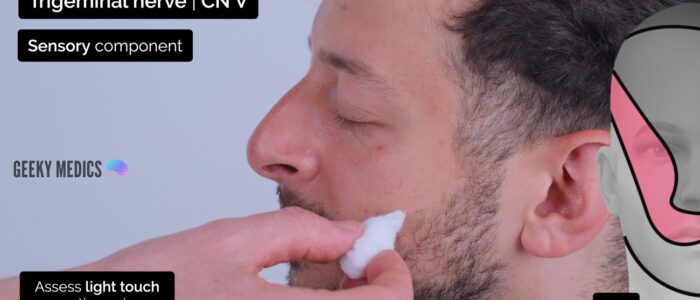
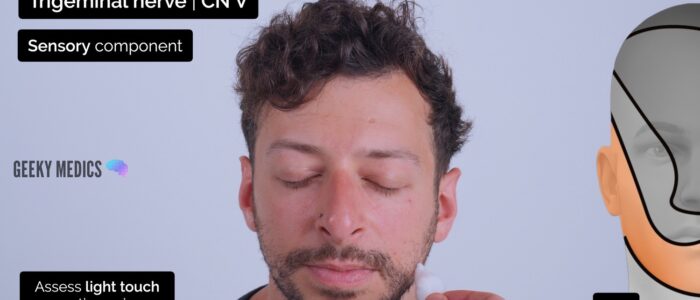
Assess the sensory component of V1, V2 and V3 by testing light touch and pinprick sensation across regions of the face supplied by each branch:
- Forehead (lateral aspect): ophthalmic (V1)
- Cheek: maxillary (V2)
- Lower jaw (avoid the angle of the mandible as it is supplied by C2/C3): mandibular branch (V3)
You should compare each region on both sides of the face to allow the patient to identify subtle differences in sensation.
Motor assessment
Use the muscles of mastication to assess the motor component of V3:
1. Inspect the temporalis (located in the temple region) and masseter muscles (located at the posterior jaw) for evidence of wasting. This is typically most noticeable in the temporalis muscles, where a hollowing effect in the temple region is observed.
2. Palpate the masseter muscle (located at the posterior jaw) bilaterally whilst asking the patient to clench their teeth to allow you to assess and compare muscle bulk.
3. Ask the patient to open their mouth whilst you apply resistance underneath the jaw to assess the lateral pterygoid muscles. An inability to open the jaw against resistance or deviation of the jaw (typically to the side of the lesion) may occur in trigeminal nerve palsy.
Reflexes
Jaw jerk reflex
The jaw jerk reflex is a stretch reflex that involves the slight jerking of the jaw upwards in response to a downward tap. This response is exaggerated in patients with an upper motor neuron lesion. Both afferent and efferent pathways of the jaw jerk reflex involve the trigeminal nerve.
To assess the jaw jerk reflex:
1. Clearly explain what the procedure will involve to the patient and gain consent to proceed.
2. Ask the patient to open their mouth.
3. Place your finger horizontally across the patient’s chin.
4. Tap your finger gently with the tendon hammer.
5. In healthy individuals, this should trigger a slight closure of the mouth. In patients with upper motor neuron lesions, the jaw may briskly move upwards causing the mouth to close completely.
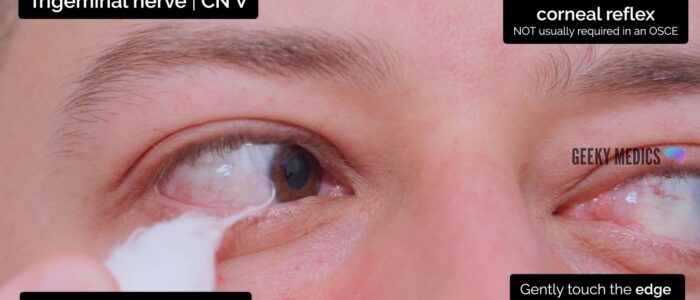
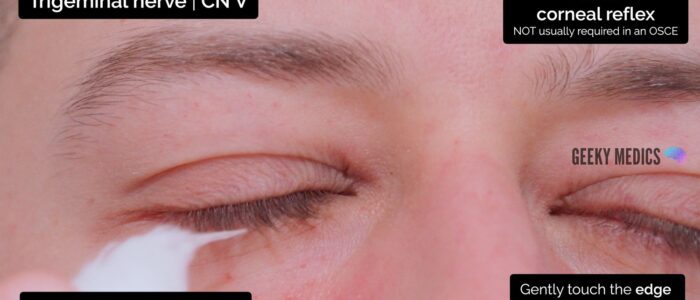
Corneal reflex
The corneal reflex involves involuntary blinking of both eyelids in response to unilateral corneal stimulation (direct and consensual blinking). The afferent branch of the corneal reflex involves V1 of the trigeminal nerve whereas the efferent branch is mediated by the temporal and zygomatic branches of the facial nerve.
To assess the corneal reflex:
1. Clearly explain what the procedure will involve to the patient and gain consent to proceed.
2. Ask the patient to turn their eyes away from the direction you are going to approach (e.g. if you’re on the patient’s right, ask them to look to the left).
3. The sclera shares the same innervation as the cornea (V1) and thus, to reduce the risk of causing corneal damage it is reasonable to first touch the sclera with a wisp of cotton wool to assess the reflex. If there is no response, repeat the assessment by gently touching the edge of the cornea (the sclera may be less sensitive, particularly in patients who wear contact lenses).
4. In healthy individuals, you should observe both direct and consensual blinking. The absence of a blinking response suggests pathology involving either the trigeminal or facial nerve.
The corneal reflex is not usually assessed in an OSCE scenario, however, you should offer to test it and understand the purpose behind the test.
Facial nerve (CN VII)
The facial nerve (CN VII) transmits motor information to the muscles of facial expression and the stapedius muscle (involved in the regulation of hearing). The facial nerve also has a sensory component responsible for the conveyance of taste from the anterior two-thirds of the tongue.
Sensory assessment
Ask the patient if they have noticed any recent changes in their sense of taste.
Motor assessment
Hearing changes
Ask the patient if they have noticed any changes to their hearing (paralysis of the stapedius muscle can result in hyperacusis).
Inspection
Inspect the patient’s face at rest for asymmetry, paying particular attention to:
- Forehead wrinkles
- Nasolabial folds
- Angles of the mouth
Facial movement
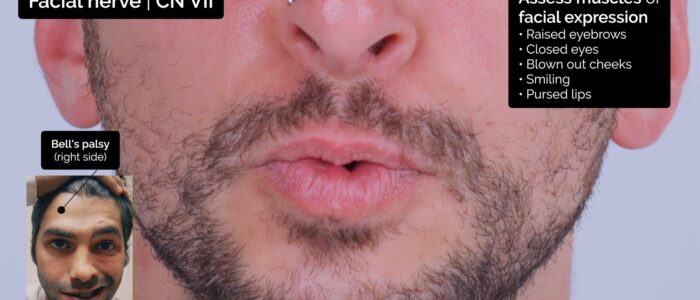
Ask the patient to carry out a sequence of facial expressions whilst again observing for asymmetry:
- Raised eyebrows: assesses frontalis – “Raise your eyebrows as if you’re surprised.”
- Closed eyes: assesses orbicular oculi – “Scrunch up your eyes and don’t let me open them.”
- Blown out cheeks: assesses orbicularis oris – “Blow out your cheeks and don’t let me deflate them.”
- Smiling: assesses levator anguli oris and zygomaticus major – “Can you do a big smile for me?”
- Pursed lips: assesses orbicularis oris and buccinator – “Can you try to whistle?”
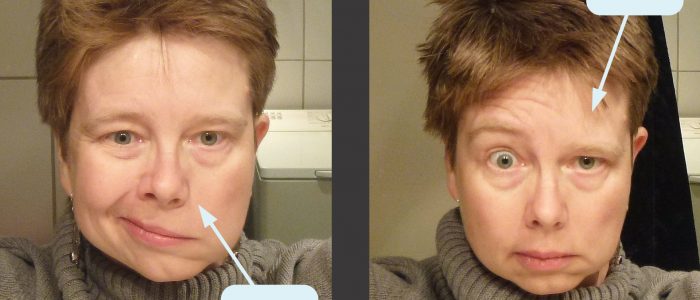
Facial nerve palsy
Facial nerve palsy presents with unilateral weakness of the muscles of facial expression and can be caused by both upper and lower motor neuron lesions.
Facial nerve palsy caused by a lower motor neuron lesion presents with weakness of all ipsilateral muscles of facial expression, due to the loss of innervation to all muscles on the affected side. The most common cause of lower motor neuron facial palsy is Bell’s palsy.
Facial nerve palsy caused by an upper motor neuron lesion also presents with unilateral facial muscle weakness, however, the upper facial muscles are partially spared because of bilateral cortical representation (resulting in forehead/frontalis function being somewhat maintained). The most common cause of upper motor neuron facial palsy is stroke.
Vestibulocochlear nerve (CN VIII)
The vestibulocochlear nerve (CN VIII) transmits sensory information about sound and balance from the inner ear to the brain. The vestibulocochlear nerve has no motor component.
Gross hearing assessment
Preparation
Ask the patient if they have noticed any change in their hearing recently.
Explain that you’re going to say 3 words or 3 numbers and you’d like the patient to repeat them back to you (choose two-syllable words or bi-digit numbers).
Assessment
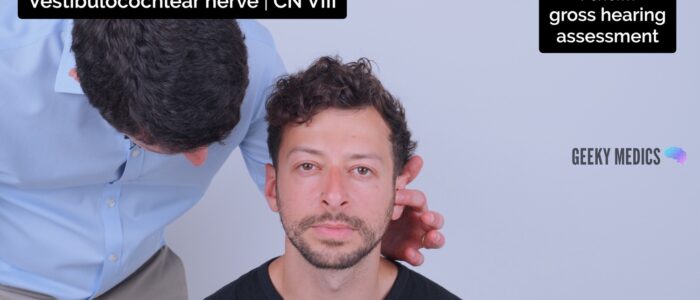
1. Position yourself approximately 60cm from the ear and then whisper a number or word.
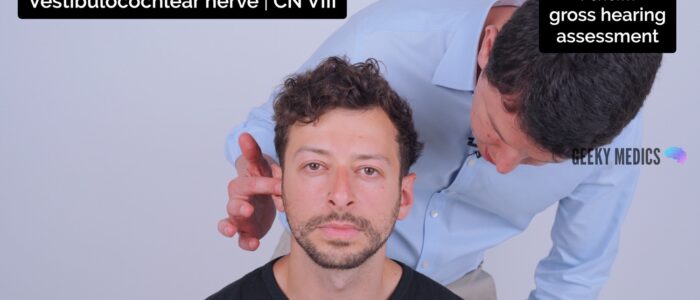
2. Mask the ear not being tested by rubbing the tragus. Do not place your arm across the face of the patient when rubbing the tragus, it is far nicer to occlude the ear from behind the head. If possible shield the patient’s eyes to prevent any visual stimulus.
3. Ask the patient to repeat the number or word back to you. If they get two-thirds or more correct then their hearing level is 12db or better. If there is no response use a conversational voice (48db or worse) or loud voice (76db or worse).
4. If there is no response you can move closer and repeat the test at 15cm. Here the thresholds are 34db for a whisper and 56db for a conversational voice.
5. Assess the other ear in the same way.
Rinne’s test
1. Place a vibrating 512 Hz tuning fork firmly on the mastoid process (apply pressure to the opposite side of the head to make sure the contact is firm). This tests bone conduction.
2. Confirm the patient can hear the sound of the tuning fork and then ask them to tell you when they can no longer hear it.
3. When the patient can no longer hear the sound, move the tuning fork in front of the external auditory meatus to test air conduction.
4. Ask the patient if they can now hear the sound again. If they can hear the sound, it suggests air conduction is better than bone conduction, which is what would be expected in a healthy individual (this is often confusingly referred to as a “Rinne’s positive” result).
Summary of Rinne’s test results
These results should be assessed in context with the results of Weber’s test before any diagnostic assumptions are made:
- Normal result: air conduction > bone conduction (Rinne’s positive)
- Sensorineural deafness: air conduction > bone conduction (Rinne’s positive) – due to both air and bone conduction being reduced equally
- Conductive deafness: bone conduction > air conduction (Rinne’s negative)
Weber’s test
1. Tap a 512Hz tuning fork and place in the midline of the forehead. The tuning fork should be set in motion by striking it on your knee (not the patient’s knee or a table).
2. Ask the patient “Where do you hear the sound?”
These results should be assessed in context with the results of Rinne’s test before any diagnostic assumptions are made:
- Normal: sound is heard equally in both ears.
- Sensorineural deafness: sound is heard louder on the side of the intact ear.
- Conductive deafness: sound is heard louder on the side of the affected ear.
A 512Hz tuning fork is used as it gives the best balance between time of decay and tactile vibration. Ideally, you want a tuning fork that has a long period of decay and cannot be detected by vibration sensation.
Conductive vs sensorineural hearing loss
Conductive hearing loss occurs when sound is unable to effectively transfer at any point between the outer ear, external auditory canal, tympanic membrane and middle ear (ossicles). Causes of conductive hearing loss include excessive ear wax, otitis externa, otitis media, perforated tympanic membrane and otosclerosis.
Sensorineural hearing loss occurs due to dysfunction of the cochlea and/or vestibulocochlear nerve. Causes of sensorineural hearing loss include increasing age (presbycusis), excessive noise exposure, genetic mutations, viral infections (e.g. cytomegalovirus) and ototoxic agents (e.g. gentamicin).
Vestibular testing – “Unterberger” or “Turning test”
Ask the patient to march on the spot with their arms outstretched and their eyes closed:
- Normal result: the patient remains in the same position.
- Vestibular lesion: the patient will turn towards the side of the lesion
Vestibular testing – “Head thrust test” or “Vestibular-ocular reflex”
Before performing this test you need to check if the patient has any neck problems and if so you should not proceed.
1. Explain to the patient that the test will involve briskly turning their head and then gain consent to proceed.
2. Sit facing the patient and ask them to fixate on your nose at all times during the test.
3. Hold their head in your hands (one hand covering each ear) and rotate it rapidly to the left, at a medium amplitude.
4. Repeat this process, but this time turn the head to the right.
The normal response is that ocular fixation is maintained. In a patient with loss of vestibular function on one side, the eyes will first move in the direction of the head (losing fixation), before a corrective refixation saccade occurs towards your nose.
Glossopharyngeal (CN IX) and vagus (CN X) nerves
The glossopharyngeal nerve transmits motor information to the stylopharyngeus muscle which elevates the pharynx during swallowing and speech. The glossopharyngeal nerve also transmits sensory information that conveys taste from the posterior third of the tongue. Visceral sensory fibres of CN IX also mediate the afferent limb of the gag reflex.
The vagus nerve transmits motor information to several muscles of the mouth which are involved in the production of speech and the efferent limb of the gag reflex.
The glossopharyngeal and vagus nerves are assessed together because of their closely related functions.
Assessment
Ask the patient if they have experienced any issues with swallowing, as well as any changes to their voice or cough.
Inspection
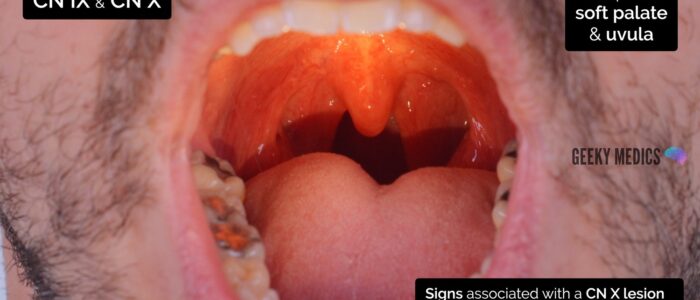
Ask the patient to open their mouth and inspect the soft palate and uvula:
- Note the position of the uvula. Vagus nerve lesions result in deviation of the uvula towards the unaffected side.
Ask the patient to say “ahh“:
- Inspect the palate and uvula which should elevate symmetrically, with the uvula remaining in the midline. A vagus nerve lesion will cause asymmetrical elevation of the palate and uvula deviation away from the lesion.
Ask the patient to cough:
- Vagus nerve lesions can result in the presence of a weak, non-explosive sounding bovine cough caused by an inability to close the glottis.
Swallow assessment
Ask the patient to take a small sip of water (approximately 3 teaspoons) and observe the patient swallow. The presence of a cough or a change to the quality of their voice suggests an ineffective swallow which can be caused by both glossopharyngeal (afferent) and vagus (efferent) nerve pathology.
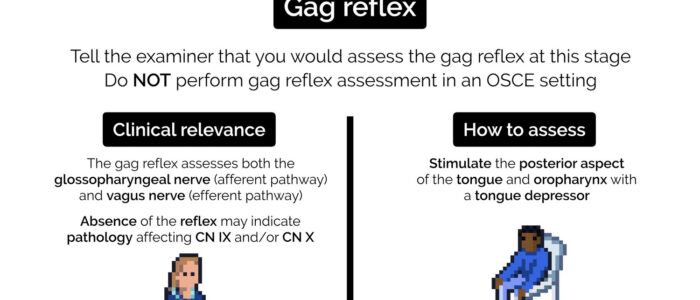
Gag reflex
The gag reflex involves both the glossopharyngeal nerve (afferent) and the vagus nerve (efferent). This test is highly unpleasant for patients and therefore the swallow test mentioned previously is preferred as an alternative. You should not perform this test in an OSCE, although you may be expected to have an understanding of what cranial nerves are involved in the reflex.
To perform the gag reflex:
1. Stimulate the posterior aspect of the tongue and oropharynx which in healthy individuals should trigger a gag reflex. The absence of a gag reflex can be caused by both glossopharyngeal and vagus nerve pathology.
Accessory nerve (CN XI)
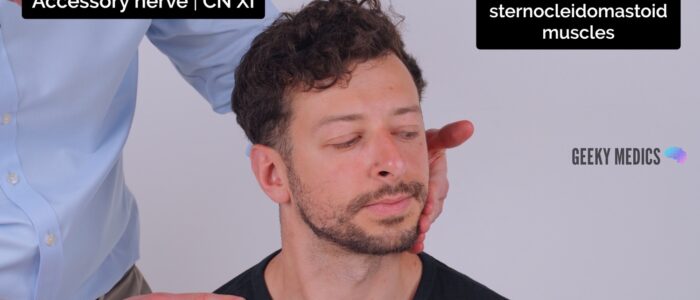
The accessory nerve (CN XI) transmits motor information to the sternocleidomastoid and trapezius muscles. It does not have a sensory component.
Assessment
To assess the accessory nerve:
1. First, inspect for evidence sternocleidomastoid or trapezius muscle wasting.
2. Ask the patient to raise their shoulders and resist you pushing them downwards: this assesses the trapezius muscle (accessory nerve palsy will result in weakness).
3. Ask the patient to turn their head left whilst you resist the movement and then repeat with the patient turning their head to the right: this assesses the sternocleidomastoid muscle (accessory nerve palsy will result in weakness).
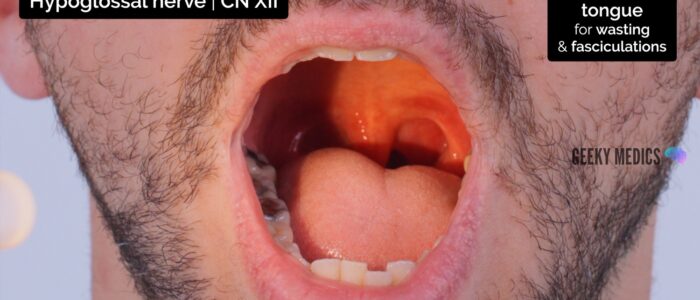
Hypoglossal nerve (CN XII)
The hypoglossal nerve (CN XII) transmits motor information to the extrinsic muscles of the tongue (except for palatoglossus which is innervated by the vagus nerve). It does not have a sensory component.
Assessment
To assess the hypoglossal nerve:
1. Ask the patient to open their mouth and inspect the tongue for wasting and fasciculations at rest (minor fasciculations can be normal).
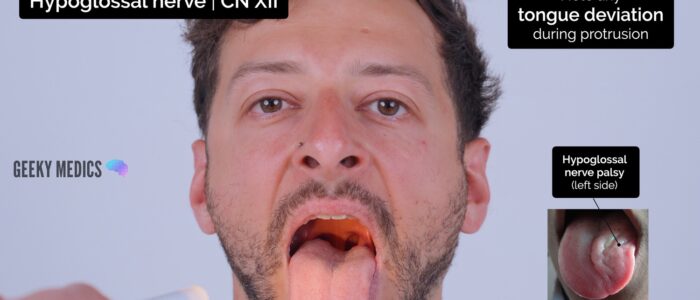
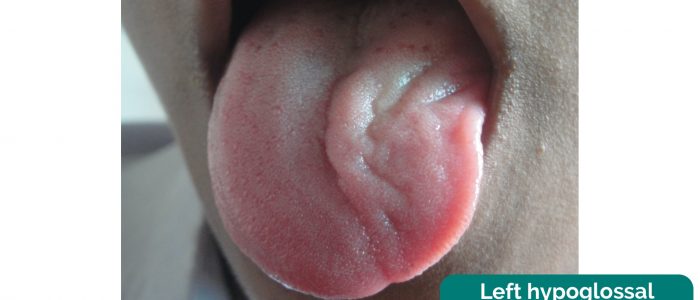
2. Ask the patient to protrude their tongue and observe for any deviation (which occurs towards the side of a hypoglossal lesion).
3. Place your finger on the patient’s cheek and ask them to push their tongue against it. Repeat this on each cheek to assess and compare power (weakness would be present on the side of the lesion).
Hypoglossal nerve palsy
Hypoglossal nerve palsy causes atrophy of the ipsilateral tongue and deviation of the tongue when protruded towards the side of the lesion. This occurs due to the overaction of the functioning genioglossus muscle on the unaffected side of the tongue.
To complete the examination…
Explain to the patient that the examination is now finished.
Thank the patient for their time.
Dispose of PPE appropriately and wash your hands.
Summarise your findings.
Example summary
“Today I examined Mrs Smith, a 64-year-old female. On general inspection, the patient appeared comfortable at rest, with normal speech and no other stigmata of neurological disease. There were no objects or medical equipment around the bed of relevance.”
“Examination of all twelve cranial nerves was unremarkable.”
“In summary, these findings are consistent with a normal cranial nerve examination.”
“For completeness, I would like to perform the following further assessments and investigations.”
Further assessments and investigations
- Full neurological examination including the upper and lower limbs.
- Neuroimaging (e.g. MRI head): if there are concerns about space-occupying lesions or demyelination.
- Formal hearing assessment (including pure tone audiometry): if there are concerns about vestibulocochlear nerve function.
Reviewer
Dr David Bargiela
Neurologist
References
- Jonathan Trobe, M.D. Adapted by Geeky Medics. RAPD. Licence: CC BY.
- Andrea Kamphuis. Adapted by Geeky Medics. Bell’s palsy. Licence: [CC BY-SA 4.0].
- Andrea Kamphuis. Adapted by Geeky Medics. Bell’s palsy. Licence: [CC BY-SA 4.0].
- Mukherjee SK, Gowshami CB, Salam A, Kuddus R, Farazi MA, Baksh J. Adapted by Geeky Medics. Hypoglossal nerve palsy. Licence: CC BY.