- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
In the United Kingdom, approximately 366,000 cases of cancer were diagnosed in 2017. It is estimated that 1 in 2 people in the UK will be diagnosed with a form of cancer in their lifetime.1
Chemotherapy agents are commonly used in the management of cancer, both in the curative and palliative setting.
Most clinicians will, at some point, encounter patients receiving chemotherapy agents and it is therefore important to have some basic understanding of their mechanism and potential side effects.
The cell cycle
The mechanism of action of chemotherapy agents typically involves disrupting various parts of the cell cycle.
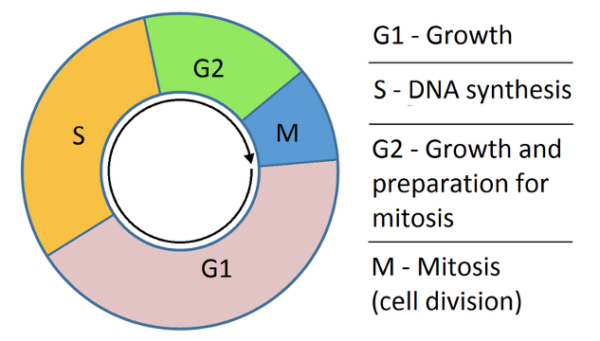
The cell replication cycle via mitosis can be divided into four main stages.
A 5th stage (G0) is not shown in the diagram and is the resting phase for the cell. This is less relevant as none of the chemotherapy agents affects cells in the G0 phase.
The four main phases of the cell replication cycle are:3
- Growth phase 1 (G1): cell growth. The cell is metabolically active and protein synthesis occurs. No DNA replication occurs within this phase.
- S phase: synthesis stage where DNA replication occurs. DNA helicase unwinds the DNA strands and DNA polymerase extends the DNA chain. Topoisomerase prevents the supercoiling of DNA during the cell replication process.
- Growth phase 2 (G2): cell growth and protein synthesis in preparation for mitosis.
- M phase: mitosis
Mitosis can be further divided into five phases:
- Prophase: condensation of chromosomes and the beginning of the formation of microtubules. Centromeres migrate to the poles of the cell to form mitotic spindles.
- Prometaphase: nuclear membrane breakdown and attachment of microtubules to the chromosomal kinetochores.
- Metaphase: chromosomes migrate to the equatorial centre of the cells with centromeres at the poles of the cells.
- Anaphase: separation of daughter chromatids by depolymerisation of microtubules.
- Telophase: reassembly of the nuclear membrane with the disassembly of microtubule complexes. Decondensation of chromosomes occurs.
Side effects of chemotherapy
Most chemotherapy agents are non-specific and will target both healthy and cancerous cells.
As these agents act on rapidly dividing cells, certain groups of cells are disproportionately affected.
Bone marrow
Haematopoietic suppression is common due to the rapid turnover of cells involved in haematopoiesis.
Clinically, patients develop anaemia, thrombocytopenia and leukopenia.
Neutropenic sepsis
Neutropenic sepsis is defined by NICE as a neutrophil count of 0.5 × 109 per litre or lower, plus one of the following:
- Temperature ≥ 38°C or
- Other signs or symptoms consistent with significant sepsis
Neutropenic sepsis is the most common medical emergency amongst oncology and haematology patients, who can present with, or rapidly progress to haemodynamic instability. Therefore, rapid assessment and administration of empirical antibiotic therapy can be lifesaving.
Gastrointestinal and dermatological
Increased apoptosis within the rapidly dividing epithelial cells in the oral mucosa and intestine causes mucosal damage throughout the gastrointestinal tract, causing mucositis, malabsorption and diarrhoea.
The direct effect of some chemotherapy agents on the chemoreceptor trigger zone within the medulla can cause severe nausea and vomiting.
Chemotherapy-induced damage to the rapidly dividing cells in the hair root results in hair loss.
Classes of chemotherapy agents
There are several methods of classifying chemotherapy agents. It is possible to classify them by the origin of the medication, mechanism of action or via the site of action.4
This article will classify chemotherapy agents by their mechanism of action. While not all chemotherapy agents are covered here, this should cover the most common chemotherapy agents. Newer monoclonal antibody therapies are not included in this guide.
Antimetabolites
Antimetabolites act by replacing the natural building materials needed for DNA and RNA synthesis. This prevents DNA replication, halting cell reproduction.4,5,6
Antimetabolites act on the S phase of the cell cycle.7
Pyrimidine antagonists8
Pyrimidine antagonists inhibit thymidylate synthase. This prevents the synthesis of the thymidine nucleotide, halting DNA replication.
Examples of agents in this class include:
- Gemcitabine: used in pancreatic and ovarian cancer
- 5-FU (and associated prodrug capecitabine): used in colorectal, gastric and pancreatic cancers
- Cytarabine: used in lymphoma and leukaemia
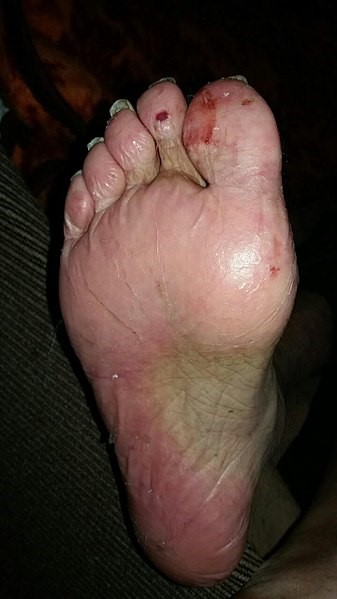
A specific side effect associated with this class of agents is palmar-plantar erythrodysesthesia (PPE).
Palmar plantar erythrodysesthesia (PPE)
PPE is a painful erythematous rash occurring over the palms, fingers and soles of the feet. Lesions are typically clearly demarcated and painful. Skin breakdown and blistering typically develop as the condition progresses.
This is typically a dose-related side effect.
Management of the rash is supportive and includes the use of emollients and steroid creams to reduce inflammation.
Purine antagonists8
Purine antagonists inhibit enzymes used in the production of purines, preventing the synthesis of adenosine and guanine.
Examples of agents in this class include:
- 6-mercaptopurine and prodrug azathioprine: used in acute lymphocytic leukaemia
- Fludarabine: commonly used in low-grade lymphomas
Measurement of thiopurine methyltransferase (TMPT) levels should be performed before prescribing 6-MP and azathioprine. A deficiency of this enzyme indicates an increased risk of toxicity.
Similarly, these agents should not be used with xanthine oxidase inhibitors (e.g. allopurinol) as both medications require xanthine oxidase for their metabolism. Concurrent use of both medications risks mercaptopurine toxicity and myelosuppression.
Folate antagonists8
The most common agent in this class is methotrexate. Folate antagonists inhibit dihydrofolate reductase and thymidine synthase, preventing the synthesis of the thymine nucleotide. Methotrexate is commonly used in lymphoma, leukaemia and choriocarcinoma.
Specific side effects include hepatic and pulmonary fibrosis. These side effects are monitored using baseline chest X-ray and pulmonary function tests before initiation of medication along with regular monitoring of liver function tests. Folic acid administration on days off methotrexate can reduce side effects.
Ribonucleotide reductase inhibitors10
Hydroxyurea is an agent in this class used in chronic myeloid leukaemia. It acts by inhibiting ribonucleotide reductase, preventing the formation of deoxyribonucleotides. The main side effect is myelosuppression.
Alkylating agents11,12,13
Alkylating agents act directly on DNA strands and cause cross-linking of DNA and RNA strands. This results in permanent modification of the DNA structure leading to programmed cell death.
These agents act on multiple phases of the cell cycle. All of these agents are known to cause significant myelosuppression.
Cyclophosphamide
Cyclophosphamide is typically used in the management of breast, ovarian and small cell lung cancer. It can also be used as part of the combination chemotherapy regime in leukaemia and lymphoma.
Side effects include bladder and pulmonary toxicity. Haemorrhagic cystitis can occur and severity can differ from mild haematuria to frank haematuria with clot formation. Transitional cell cancer of the bladder has also been associated with cyclophosphamide.
Platinum agents
Examples of platinum agents include cisplatin, carboplatin and oxaliplatin. Each agent contains a platinum ion, surrounded by organic ligands, that carries out its action on DNA strands. These agents are commonly used in the management of ovarian, colorectal, lung and bladder cancers.
Significant side effects include reversible peripheral neuropathy and nephrotoxicity. Renal function should be closely monitored when these agents are used.
Nitrogen mustards
Chlorambucil and melphalan are common agents in this class. Chlorambucil is used in Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Chlorambucil can cause pulmonary fibrosis.
Melphalan is used in multiple myeloma and is also associated with pulmonary toxicities including pulmonary fibrosis and oedema.
Topoisomerase inhibitors14,15
These are natural plant extracts that inhibit the topoisomerase family of enzymes.
Topoisomerase I inhibition results in the breakdown of single-stranded DNA while topoisomerase II inhibition results in the breakdown of double-stranded DNA. Both result in cell cycle arrest.
Agents in this class inhibit cell division during the S and G2 phase.
Common side effects of medications in this class include myelosuppression, alopecia and mucositis.
Topoisomerase I inhibitors
Examples of these agents include irinotecan and topotecan. Irinotecan is commonly used in colorectal, pancreatic and small cell lung cancer. Topotecan is used in cervical, ovarian and small cell lung cancer.
Pulmonary toxicities, including pneumonitis, are rare side effects of topoisomerase I inhibitors.
Topoisomerase II inhibitors
Etoposide is an example agent in this class. It is commonly used in testicular cancer and leukaemia.
Apart from the common side effects of this class, it is not associated with any other specific side effects.
Mitotic inhibitors16
Mitotic inhibitors act on the microtubules that form during cell replication. They disrupt their structure and prevent the cell cycle from progressing past the metaphase stage.
These agents can cause myelosuppression and neurotoxicity (predominantly peripheral neuropathy).
Vinca alkaloids
Examples of agents in this class include vincristine, vinblastine and vinorelbine. These agents are commonly used in solid tumours (including Wilms tumour and neuroblastomas) and lymphomas (Hodgkin’s and non-Hodgkin’s).
Vinca alkaloids act by binding on the b-tubulin units and prevents microtubule polymerization. This prevents spindle formation and the cell cycle arrests during metaphase.
Taxanes
Agents in this class include docetaxel and paclitaxel. These are commonly used in breast, ovarian and gastric cancers.
Taxanes bind to polymerised microtubules and cause hyperstabilisation of the microtubule structure. Lack of microtubule depolymerization prevents chromosomal separation, leading to cell cycle arrest in metaphase.
Nail changes including increased pigmentation, nail bed purpura and splinter haemorrhages can develop with the use of taxanes.
Antibiotics
There are a diverse class of chemotherapy agents that are derived from antibiotic agents.
Mitomycin C17
This antibiotic works by alkylating DNA strands causing DNA damage and preventing further DNA replication.
Mitomycin C is used in bladder cancer and was previously used in gastric cancer before the introduction of more modern chemotherapy agents.
Side effects include haemolytic uraemic syndrome, myelosuppression and cardiomyopathy.
Bleomycin18
Bleomycin is commonly used in testicular cancer and Hodgkin’s lymphoma. It acts by binding to metallic ions forming complexes that generate reactive oxygen species.
This induced oxidative damage to the DNA strands, halting the cell cycle in the G2 phase.
Bleomycin is unique as it has minimal impact on myelosuppression but is associated with mucositis. Its most serious side effect is pulmonary fibrosis, and this limits its use.
Anthracyclines19,20
Examples of anthracyclines include doxorubicin, epirubicin and idarubicin. They are commonly used in breast cancer and non-Hodgkin’s lymphoma but are also used as part of leukaemia treatment regimes.
Anthracyclines have multiple modes of action including the formation of free radicals causing oxidative DNA damage and stabilisation of the topoisomerase II-DNA complex, preventing DNA synthesis.
Anthracyclines are associated with myelosuppression, alopecia and cardiomyopathy.
Cardiomyopathy can occur during the acute phase of chemotherapy administration or present years following chemotherapy administration. Care should be taken when administrating other cardiotoxic agents as this can induce cardiomyopathy.
Echocardiography is used in this case to detect early cases of cardiomyopathy.
Protein kinase inhibitors21,22,23,24
This is a diverse class of medication that targets protein kinases. A protein kinase is an enzyme that phosphorylates a target protein. These target proteins are often involved in the cell signalling pathway triggering cell division.
Each protein kinase inhibitor is named after the enzymes it inhibits.
BCR-ABL tyrosine kinase inhibitors
Examples in this class include imatinib and which is commonly used in the management of chronic myeloid leukaemia.
Side effects of imatinib include neurotoxicity, hepatotoxicity and nephrotoxicity.
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors
Erlotinib and gefitinib are examples of these agents. They are typically used in the management of lung cancer. Erlotinib can also be used in pancreatic cancers.
Side effects from this class include skin toxicity (presenting as folliculitis), pulmonary fibrosis and diarrhoea.
Brunton kinase inhibitors
Ibrutinib is in this class and is used in the management of chronic lymphocytic leukaemia. It is associated with cardiotoxicity, hepatotoxicity and myelosuppression.
Multiple receptor kinase inhibitors
These agents inhibit multiple receptors including vascular endothelial growth factor receptors (VEGFR) and platelet-derived growth factor receptors (PDGFR). Examples of these agents include sunitinib and pazopanib.
Sunitinib is used in the management of gastrointestinal stromal tumours, renal cancer and pancreatic cancer. Pazopanib is used in renal cancer.
Side effects include diarrhoea and nephrotoxicity (rashes and alopecia can also occur).
Editor
Dr Chris Jefferies
References
- World Cancer Research Fund. Cancer Statistics. Available from: [LINK]
- Simon Caulton. Cell replication cycle. License: [CC-BY-SA]. Available from: [LINK]
- Cooper, G.M. The Eukaryotic Cell Cycle. Published in 2019. Available from: [LINK]
- SEER Training. Types of Chemotherapy Drugs. Available from: [LINK]
- American Cancer Society. How Chemotherapy Drugs Work. Published in 2013. Available from: [LINK]
- Cancer Network. Principles of Oncologic Pharmacotherapy. Available from: [LINK]
- Cleveland Clinic Cancer. Types of Chemotherapy – What is Chemotherapy? . Published in 2002. Available from: [LINK]
- Chemoth. Antimetabolites. Available from: [LINK]
- Nudeldorf MSV. Foot on capeciatbine. License: [CC-BY-SA]. Available from: [LINK]
- Jinna, S and Khandhar, P.B. Hydroxyurea Toxicity. Published in 2020. Available from: [LINK]
- Colvin, M. Alkylating Agents. Published in 2017. Available from: [LINK]
- Hall, A.G. and Tilby, M.J. (1992). Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Reviews, 6(3), pp.163–173
- Ogino, M.H. and Tadi, P. Cyclophosphamide. Published in 2020. Available from: [LINK]
- LiverTox. Topoisomerase Inhibitors. Published in 2012. Available from: [LINK]
- chemoth.com. Topoisomerase Inhibitors. (n.d.). Available from: [LINK]
- van Vuuren, R.J., Visagie, M.H., Theron, A.E. and Joubert, A.M. (2015). Antimitotic drugs in the treatment of cancer. Cancer Chemotherapy and Pharmacology, 76(6), pp.1101–1112.
- Verweij J, Pinedo HM. Mitomycin C: Mechanism of action, usefulness and limitation. Available from: [LINK]
- Brandt, J.P. and Gerriets, V. Bleomycin. Published in 2020. Available at: [LINK]
- Venkatesh, P. and Kasi, A. Anthracyclines. Published in 2020. Available at: [LINK]
- chemoth.com. Anthracycline Chemotherapy Agents.. Available from: [LINK]
- Gross, S., Rahal, R., Stransky, N., Lengauer, C. and Hoeflich, K.P. (2015). Targeting cancer with kinase inhibitors. Journal of Clinical Investigation. 125(5), pp.1780–1789. Available from: [LINK]
- Kannaiyan, R. and Mahadevan, D. (2018). A comprehensive review of protein kinase inhibitors for cancer therapy. Expert review of anticancer therapy. 18(12), pp.1249–1270. Available from: [LINK]
- Bhullar, K.S., Lagarón, N.O., McGowan, E.M., Parmar, I., Jha, A., Hubbard, B.P. and Rupasinghe, H.P.V. (2018). Kinase-targeted cancer therapies: progress, challenges and future directions. Molecular Cancer. 17(1). Available from: [LINK].
- Widakowich, C., de Castro, G., de Azambuja, E., Dinh, P. and Awada, A. (2007). Review: Side Effects of Approved Molecular Targeted Therapies in Solid Cancers. The Oncologist, 12(12), pp.1443–1455.




