- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Anaemia is a common condition that results in impaired oxygen delivery to body tissues due to reduced haemoglobin. It affects over 1.62 billion people globally.1,2
Anaemia is defined as a haemoglobin level two standard deviations below the normal for age and sex.3
In women, a normal haemoglobin level is 115-165 g/L, while it is slightly increased in men at 130-180 g/L, due to larger body size.4
Anaemia can be classified according to the average size of the red blood cells (RBC), referred to as mean corpuscular volume (MCV):5,6
- Microcytic anaemia: MCV < 80
- Normocytic anaemia: MCV 80 – 100
- Macrocytic anaemia: MCV >100
This article describes the general clinical features of anaemia and the causes of microcytic, normocytic and macrocytic anaemia.
Clinical features
History
Typical symptoms of anaemia include:7
- Pallor
- Fatigue
- Breathlessness
- Dizziness
- Palpitations
- Cold hands and feet
Other important areas to cover in the history include:8
- Dietary history (to rule out malnutrition)
- Causes of potential blood loss (e.g. menorrhagia)
- Issues with malabsorption (e.g. gastrectomy, coeliac disease)
- Family history of haematological disorders
- Any episodes of black tarry stools (suggestive of gastrointestinal bleeding)
- Symptoms of chronic disease (e.g. cardiac, renal, hepatic)
Clinical examination
In the context of suspected anaemia, a comprehensive examination of the major systems must be performed.
Possible clinical findings in anaemia include:9
- Pallor (e.g. general pallor or conjunctival pallor)
- Rapid or irregular heartbeat on cardiac auscultation
- Enlarged liver or spleen on palpation (consider lymphoma or leukaemia with splenomegaly)
- A pelvic or rectal exam may be indicated to assess for blood loss
Microcytic anaemias
Microcytic anaemia is defined as anaemia with an MCV of less than 80. As there is a lack of haemoglobin (Hb), an extra division of red blood cells (RBCs) occurs to maintain adequate Hb concentration.10 This results in smaller and paler (hypochromic) RBCs.
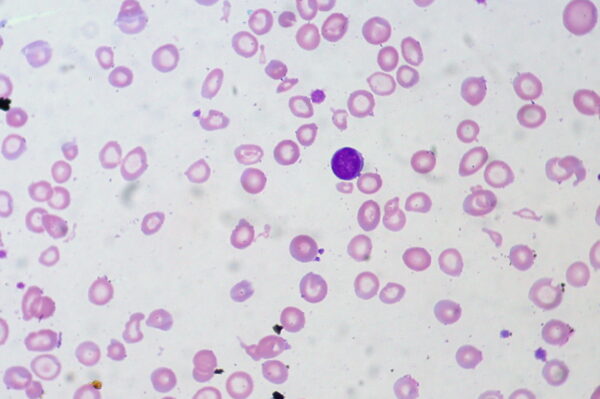
The most common cause of microcytic anaemia is iron-deficiency anaemia.
Iron deficiency anaemia
Aetiology
In iron-deficiency anaemia, there is a lack of iron, which is a vital component of haemoglobin. This condition often presents with mild symptoms (e.g. fatigue, palpitations) and can sometimes lead to strange cravings for ice, dirt, or starch.10
Iron deficiency is most often caused by chronic blood loss but can also be due to dietary deficiency, malabsorption or increased requirements during childhood or pregnancy.11
Any patient over 40 with iron deficiency anaemia requires an upper and lower gastrointestinal endoscopy. For more information, see the Geeky Medics guide to iron deficiency anaemia.
Investigation findings
Investigation findings in iron deficiency anaemia include:
- Low MCV <80
- Serum iron: low
- Transferrin saturation: low
- Ferritin: low
- Total iron-binding capacity: high (there is an inverse relationship between ferritin and TIBC)9
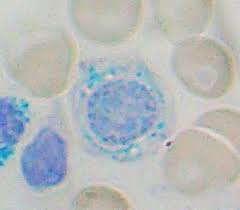
Sideroblastic anaemia
Aetiology
Sideroblastic anaemia is characterised by defective protoporphyrin synthesis. This can be due to congenital or acquired causes:
- Congenital: deficiency of aminolevulinic acid synthetase (ALAS; rate-limiting enzyme)
- Acquired: alcoholism, lead poisoning, vitamin B6 deficiency (co-factor for ALAS)
In sideroblastic anaemia, there is nothing wrong with the iron component of haemoglobin. The iron is stuck waiting around because the lack of protoporphyrin prevents the formation of haem.
This results in iron building up in mitochondria, creating the pathognomonic ringed sideroblasts (Figure 2).
Thalassaemia
Aetiology
Thalassaemias occur due to inherited mutations affecting the globin gene. They can be further classified into the specific gene that is affected (alpha or beta), as well as the severity of the condition (major, minor or trait).14
People with the thalassaemia trait may be asymptomatic or have very mild symptoms, while thalassaemia major can be severe enough to require regular transfusions.14
The most frequent occurrences of thalassaemias are in the Mediterranean, Africa, Western and Southeast Asia, as well as India and Burma.10 This condition seems to be protective against Plasmodium falciparum malaria, which is why the population distribution is so similar for the two conditions. Thalassaemias result in classic clinical features such as chipmunk facies and a crew cut appearance on X-ray.
Investigation findings
Investigation findings in thalassaemias include:15
- Low MCV <80 (usually lower relative to iron deficiency anaemia)
- Ferritin: normal (unlike the low ferritin found in iron deficiency anaemia)
- Haemoglobin electrophoresis: abnormal
- Positive genetic testing for HBB, HBA1 or HBA2
Summary table
Table 1. An overview of microcytic anaemias.
|
|
Serum iron |
% saturation |
Ferritin |
TIBC |
|
Iron deficiency anaemia |
Low |
Low |
Low (all of the stored iron is used up because serum iron is depleted) |
High |
|
Anaemia of chronic disease |
Low |
Low |
High (due to built-up stores from hepcidin) |
Low |
|
Sideroblastic anaemia |
High (iron builds up in cells and then is released into serum once the cell bursts) |
High |
High (iron overloaded state) |
Low |
Normocytic anaemias
This subset of anaemias is identified by a normal MCV of between 80-100.
Since the size of the RBCs isn’t altered, the decreased haemoglobin must be due to another cause, either haemolysis (intravascular or extravascular) or underproduction of normal-sized RBCs.
The reticulocyte count enables differentiation between the two causes. A high reticulocyte count would indicate that the bone marrow was functioning normally and therefore the anaemia is not likely to be due to underproduction.16 Unlike microcytic anaemias, normocytic anaemias are usually normochromic.
Anaemia of chronic disease
Aetiology
In anaemia of chronic disease, underlying chronic diseases such as malignancy, chronic infections, or autoimmune conditions, cause the liver to produce acute phase reactants such as hepcidin.
Hepcidin “hides” the iron in ferritin to reduce availability in the serum. Anaemia of chronic disease starts out as normocytic anaemia but it can progress to microcytic anaemia.
Investigation findings
Investigation findings in anaemia of chronic disease include:
- Normal MCV (80-100) or low MCV (<80)
- Serum iron: low
- Transferrin saturation: low
- Ferritin: high
- TIBC: low
Hereditary spherocytosis
Aetiology
Hereditary spherocytosis occurs due to an inherited defect of RBC cytoskeleton membrane proteins such as ankyrin and spectrin.
The abnormal configuration of the RBCs prevents easy manoeuvrability through the splenic infrastructure. As the RBCs get stuck, they are destroyed (haemolysed) by the spleen.
There is no issue with the RBC creating or carrying haemoglobin, the problem is that the spleen is destroying the RBCs. For this reason, splenectomy is often performed.
Investigation findings
Investigation findings in hereditary spherocytosis include:
- Reticulocyte count: increased due to compensation by the bone marrow due to extravascular haemolysis
- Serum uric acid: increased due to lysed RBCs
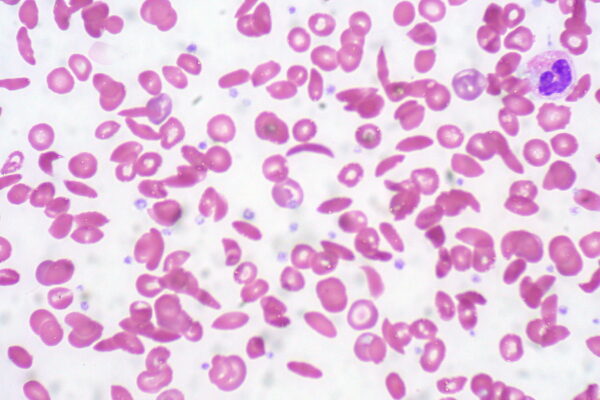
Sickle cell anaemia
Aetiology
This condition occurs due to an autosomal recessive mutation in the beta-globin chain of haemoglobin, causing valine to replace glutamic acid.
Intravascular haemolysis may occur due to the deformed sickle shape of the RBC, but the spleen has a role in the more predominant extravascular haemolysis of the misshapen cells.
For more information, see the Geeky Medics guide to sickle cell anaemia.
Investigation findings
Investigation findings in sickle cell anaemia include:
- Reticulocyte count: increased due to compensation by the bone marrow for intravascular/extravascular haemolysis
- Serum uric acid: increased due to lysed RBCs
- Blood film: sickling of erythrocytes and features of hyposplenism including target cells and Howell-Jolly bodies (Figure 3)
Paroxysmal nocturnal haemoglobinuria
Aetiology
Paroxysmal nocturnal haemoglobinuria occurs due to an acquired defect in glycosylphosphatidylinositol (GPI) that leaves cells more susceptible to the complement system.
When shallow breathing occurs during sleep, there is CO2 retention, which results in a state of mild respiratory acidosis. This environment activates complement to lyse RBCs because the protective factor (GPI) is lacking.
Investigation findings
Investigation findings in paroxysmal nocturnal haemoglobinuria include:
- Reticulocyte count: normal
- Serum uric acid: increased due to lysed RBCs
Glucose-6-phosphate dehydrogenase deficiency
Aetiology
A deficiency in glucose-6-phosphate dehydrogenase (G6PD) makes RBCs more susceptible to oxidative stress.
Normally, G6PD creates nicotinamide adenine dinucleotide phosphate (NADPH), which in turn creates reduced glutathione to protect the cell from oxidative injury. Lack of G6PD results in intravascular haemolysis due to destruction via oxidative stress.
Investigation findings
Investigation findings in G6PD deficiency include:
- Reticulocyte count: increased
- Serum uric acid: increased due to lysed RBCs
Immune haemolytic anaemia (IgG or IgM mediated)
Aetiology
IgG-mediated haemolysis is usually extravascular, while IgM-mediated haemolysis is intravascular.
Investigation findings
Investigation findings in immune haemolytic anaemia include:
- Reticulocyte count: increased due to compensation by the bone marrow for intravascular/extravascular haemolysis
- Serum uric acid: increased due to lysed RBCs
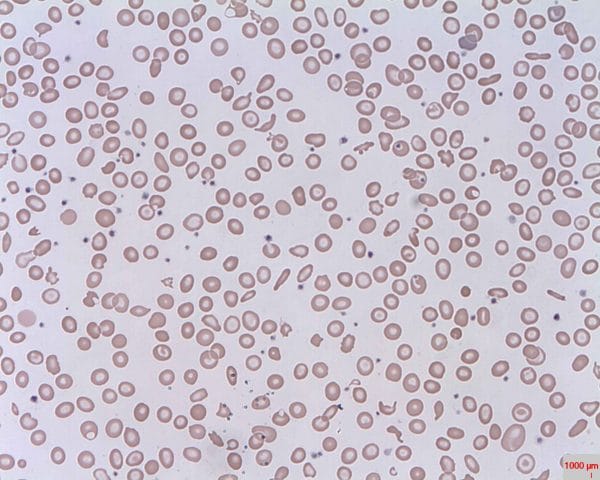
Microangiopathic haemolytic anaemia
Aetiology
In this case, RBCs are destroyed because of structural issues with the vasculature, such as microthrombi, prosthetic heart valves and aortic stenosis. Sometimes the RBCs get torn on these protruding structures, creating visibly distinct schistocytes (Figure 4).
Summary table
Table 2. An overview of normocytic anaemia.
|
|
Intravascular haemolysis |
Extravascular haemolysis |
Reticulocyte count |
Uric acid levels |
|
Hereditary spherocytosis |
|
✓ |
Increased |
Increased |
|
Sickle cell anaemia |
✓ |
✓ |
||
|
Paroxysmal nocturnal haemoglobinuria |
✓ |
|
||
|
G6PD deficiency |
✓ |
|
||
|
Immune haemolytic anaemia |
✓ (IgM) |
✓ (IgG) |
||
|
Microangiopathic haemolytic anaemia |
✓ |
|
||
|
Underproduction of RBCs |
N/A |
N/A |
Normal |
Normal |
Macrocytic anaemias
This subset of anaemias is identified by an increased MCV of over 100. They are further divided into megaloblastic and non-megaloblastic subsets.
Megaloblastic causes of macrocytic anaemia result in an increased MCV due to impaired DNA synthesis.19
Non-megaloblastic causes do not affect DNA synthesis but result in an increased MCV nonetheless.14
When trying to differentiate these causes, serum homocysteine and methylmalonic acid levels are useful investigations.
Folate deficiency (megaloblastic)
Aetiology
Folate deficiency can have multiple causes, including poor diet, increased demand (pregnancy, haemolytic anaemia, cancer), or the use of folate antagonists.20
Investigation findings
Investigation findings in folate deficiency include:
- Increased MCV (>100)
- Increased serum homocysteine
- Normal serum methylmalonic acid
Vitamin B12 deficiency (megaloblastic)
Aetiology
Vitamin B12 deficiency is the less common megaloblastic anaemia as the liver has large hepatic stores of vitamin B12 that take a while to become depleted.20
Vitamin B12 absorption requires the cofactor known as intrinsic factor, manufactured by gastric parietal cells.
In pernicious anaemia, the parietal cells are destroyed through an autoimmune process, impairing the absorption of vitamin B12.
Pernicious anaemia is the most common cause of this deficiency but pancreatic insufficiency, damage to the terminal ileum or dietary insufficiency (e.g. vegan diet) are other potential causes.
The main difference in clinical manifestation between folate and vitamin B12 deficiency are the neurological symptoms that occur in B12 deficiency, due to elevated levels of methylmalonic acid.
Investigation findings
Investigation findings in vitamin B12 deficiency include:
- Increased MCV (>100)
- Increased serum homocysteine
- Increased serum methylmalonic acid
Non-megaloblastic macrocytic anaemia
Aetiology
This type of anaemia is classified by an increased MCV that is not due to folate or vitamin B12 deficiency. Causes include alcoholism, hypothyroidism, reticulocytosis, and drugs such as fluorouracil.
Investigation findings
Investigation findings in non-megaloblastic macrocytic anaemia include:
- Increased MCV (>100)
- Normal serum homocysteine
- Normal serum methylmalonic acid
Summary table
Table 3. An overview of macrocytic anaemia.
|
|
Homocysteine levels |
Methylmalonic acid levels |
|
Folate deficiency |
↑ |
– |
|
Vitamin B12 deficiency |
↑ |
↑ |
|
Non-megaloblastic anaemia |
N/A |
N/A |
Key points
- Anaemia is defined as a haemoglobin level two standard deviations below the normal for age and sex. In women, a normal haemoglobin level is 115-165 g/L, while it is slightly increased in men at 130-180 g/L, due to larger body size.
- Anaemia is classified by the average size of RBCs: microcytic (smaller RBC size than normal), normocytic (normal RBC size) and macrocytic (larger RBC size than normal).
- Clinical features of anaemia include breathlessness, fatigue, pallor, palpitations, dizziness and cold extremities.
- Microcytic anaemias have an MCV of less than 80 and include iron deficiency anaemia, sideroblastic anaemia and thalassaemia.
- Normocytic anaemias have an MCV of between 80-100 and include anaemia of chronic disease, hereditary spherocytosis, sickle cell anaemia, paroxysmal nocturnal haemoglobinuria, G6PD deficiency, immune haemolytic anaemia, microangiopathic haemolytic anaemia and underproduction of RBCs.
- Macrocytic anaemias have an MCV of greater than 100 and include folate deficiency (megaloblastic), vitamin B12 deficiency (megaloblastic anaemia) and non-megaloblastic anaemia.
- Iron studies (serum iron, % saturation, ferritin, TIBC) can help differentiate microcytic anaemia.
- The reticulocyte count is useful for determining whether normocytic anaemia is resulting from increased destruction of RBCs or decreased production.
- Serum methylmalonic acid and homocysteine levels are useful to distinguish between subtypes of macrocytic anaemia.
Reviewer
Professor Mary Leader
MD, FRCPath., FRCPI, FFPath (RCPI), DCH, LRCP & SI
Editor
Dr Chris Jefferies
References
- Mayo Clinic Staff. Published in 2019. Available from: [LINK]
- Bruno de Benoist. Worldwide prevalence of anaemia 1993-2005. Published in 2008. Available from: [LINK]
- NICE Clinical Knowledge Summary. Anaemia- Iron Deficiency. Available from: [LINK]
- Geeky Medics. Reference Ranges. Available from: [LINK]
- Medline Plus. MCV (Mean Corpuscular Volume). Published in 2020. Available from: [LINK]
- Brittany S. Maner, Leila Moosavi. Mean Corpuscular Volume. Published in 2020. Available from: [LINK]
- Hematology-Oncology Associates of CNY. How is anemia diagnosed? Available from: [LINK]
- Joseph E. Maakaron. Anemia Clinical Presentation. Published in 2019. Available from: [LINK]
- Husain Sattar. Pathoma. Published in 2019.
- Mayo Clinic Staff. Iron deficiency anemia. Published in 2019. Available from: [LINK]
- Archana M. Agarwal. Diagnostic approach to Anemia. Published in 2014. Available from: [LINK]
- Ed Uthman. Iron-Deficiency Anaemia, Peripheral Blood Smear. License: [CC-BY].
- Paulo Henrique Orlandi Mourao. Sideroblast, as seen in sideroblastic anemia. License: [CC-BY-SA]
- Centres for Disease Control and Prevention. Thalassemia. Published in 2020. Available from: [LINK]
- Raffaella Origa. Beta-Thalassemia. Published in 2018. Available from: [LINK]
- American Association for Clinical Chemistry. Anemia. Published in 2020. Available from: [LINK]
- Ed Uthman. Sickle Cell Anemia. License: [CC-BY].
- Osaro Erhabor. Schistocytes. License: [Public domain].
- Christine A. Moore, Abdullah Adil. Macrocytic Anemia. Published in 2020. Available from: [LINK]
- NHS England. Vitamin B12 or folate deficiency anaemia. Published in 2019. Available from: [LINK]




