- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
The brain comprises around 2% of total body weight, yet it receives 15-20% of the total cardiac output. The brain has a relatively high metabolic demand due to being largely reliant on oxidative metabolism. Loss of consciousness occurs within 10 seconds of the interruption of arterial blood supply to the brain, and irreparable damage to brain tissue occurs after only a few minutes.¹
The arterial blood supply to the brain can be divided into the anterior and posterior circulation. The former is derived from the left and right internal carotid arteries, and the latter is derived from the left and right vertebral arteries.
This article will describe the arterial supply of the brain and some of the clinical consequences of disruption to this arterial supply.
The Monro-Kellie hypothesis
The cranium, enclosing the brain, forms a fixed space comprising three components: blood, cerebrospinal fluid, and brain tissue. These components remain in a state of dynamic equilibrium. Therefore any decrease in any one of them results in an increase in the other two. 2
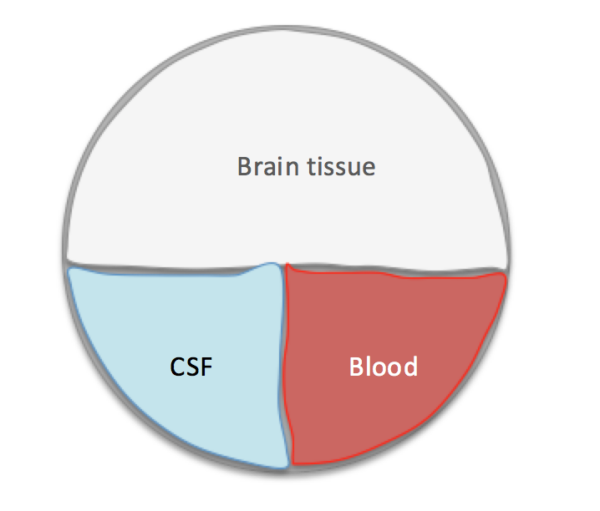
Cerebral perfusion pressure
Cerebral perfusion pressure (CPP) drives oxygen and nutrient supply to brain tissues. The brain can autoregulate blood flow to ensure a constant flow that is isolated from fluctuations in systemic blood pressure.
This microcirculation is regulated by cerebral vessel constriction and dilatation. Most of the blood within the cranial cavity is contained within the low-pressure venous system. Venous compression is the main method of displacing blood volume in the aforementioned mechanism.
This mechanism is frequently lost secondary to head trauma, leading to cerebral ischaemia and neuronal death (secondary brain injury).
Calculating CPP
CPP can be calculated using the following formula3:
CPP = MAP – ICP
Anterior circulation
The anterior circulation is responsible for supplying:
- Cerebrum
- Ophthalmic artery
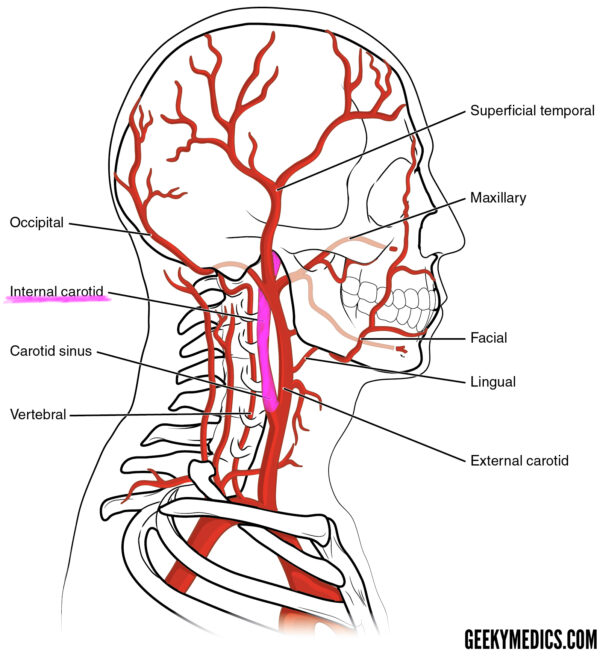
Internal carotid arteries
Course and branches
The left and right common carotid arteries bifurcate at the level of C3/C4 to give off the internal carotid arteries (ICA) within the carotid sheath.
The internal carotid arteries then proceed through the respective carotid canal, within the petrous portion of the temporal bone.
Once in the cranial cavity, the internal carotid arteries pass anteriorly through the cavernous sinus.
Once the internal carotid arteries are distal to the cavernous sinus, each gives rise to the following branches:
- Ophthalmic artery: Supplies all the structures in the orbit and some in the nose, face and meninges.
- Posterior communicating artery:
- Anteriorly connects to the internal carotid artery before the terminal bifurcation of the ICA into the anterior cerebral artery and middle cerebral artery.
- Posteriorly, it communicates with the posterior cerebral artery.
- Anterior cerebral artery: Supplies oxygenated blood to most midline portions of the frontal lobes and superior medial parietal lobes.
The internal carotid arteries then continue as the middle cerebral arteries. The middle cerebral arteries supply the lateral cerebral cortex, anterior temporal lobes and the insular cortices.
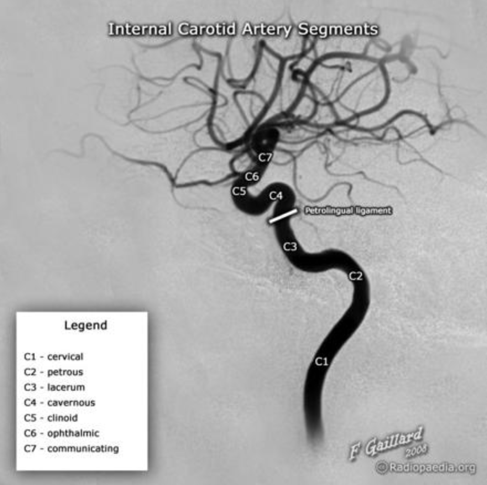
Bouthillier classification of ICA segments
This system breaks the ICA down into seven segments, which can be useful when describing pathology such as stenosis or aneurysms: 5
- C1 – Cervical
- C2 – Petrous
- C3 – Lacerum
- C4 – Cavernous
- C5 – Clinoid
- C6 – Ophthalmic (supraclinoid)
- C7 – Communicating (terminal)
Posterior circulation
The posterior circulation is responsible for supplying:
- Occipital lobes
- Cerebellum
- Brainstem
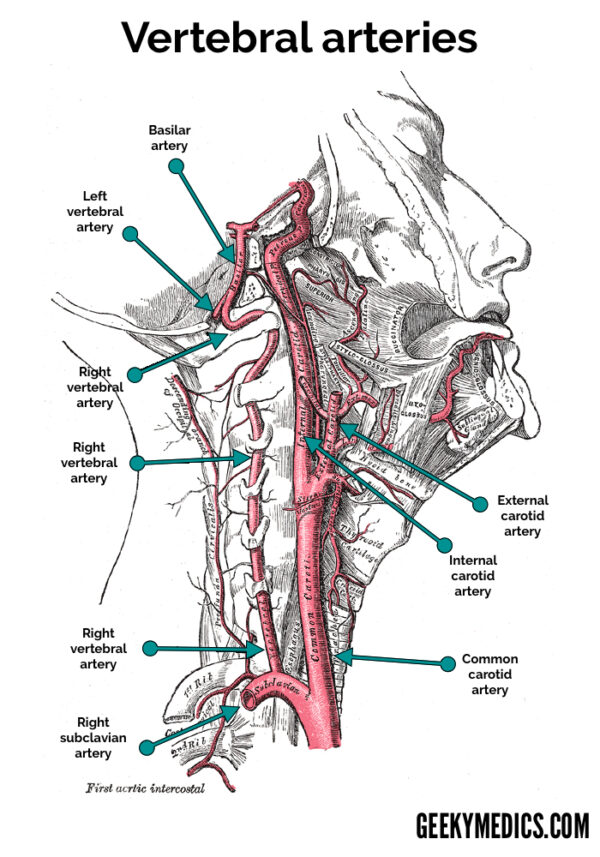
Vertebral arteries
Course and branches
The left and right vertebral arteries arise from their respective subclavian arteries, on the posterosuperior aspect.
The vertebral arteries then enter the transverse foramina of the spine at level C6 and continue superiorly.
After passing through the transverse foramen of C1, the arteries traverse the foramen magnum.
Once inside the cranial vault, the vertebral arteries give off the following branches:
- Posterior inferior cerebellar artery (PICA) – this is the largest branch of the vertebral artery and is one of three main arteries supplying the cerebellum
- Anterior and posterior meningeal arteries – supply the dura mater
- Anterior and posterior spinal arteries – supply the spinal cord along its entire length
The vertebral arteries then converge to form the basilar artery at the base of the pons, inside the cranium.
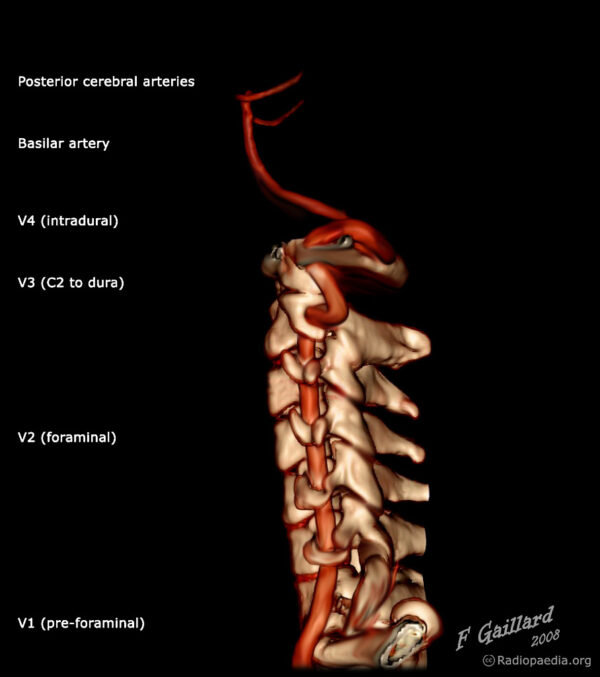
The vertebral artery is generally divided into four segments: 6
- V1 – preforaminal
- V2 – foraminal
- V3 – atlantic, extradural, or extraspinal
- V4 – intradural, intracranial
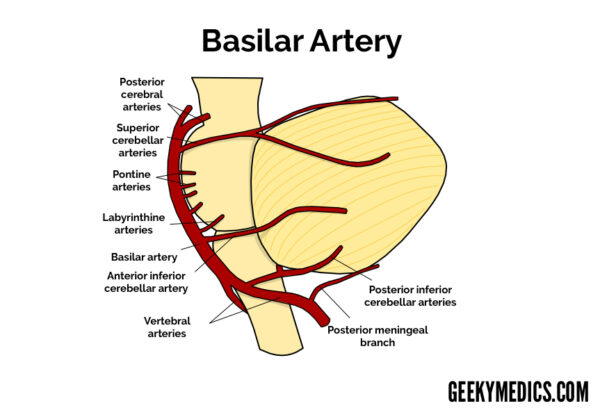
Basilar artery
Course and branches
The basilar artery runs superiorly within the central groove of the pons, giving off several branches, including the pontine arteries, which supply the pons.
The basilar artery eventually anastomoses with the circle of Willis via the posterior cerebral arteries and posterior communicating arteries.
Clinical relevance: Locked-in syndrome
Pontine infarcts cause an interruption in the myriad of neuronal pathways enabling communication between the cerebrum, cerebellum and spinal cord. This can result in complete paralysis of all voluntary muscle groups, sparing those controlling the eyes. Individuals suffering from damage to the pons are fully conscious and cognitively intact. 7
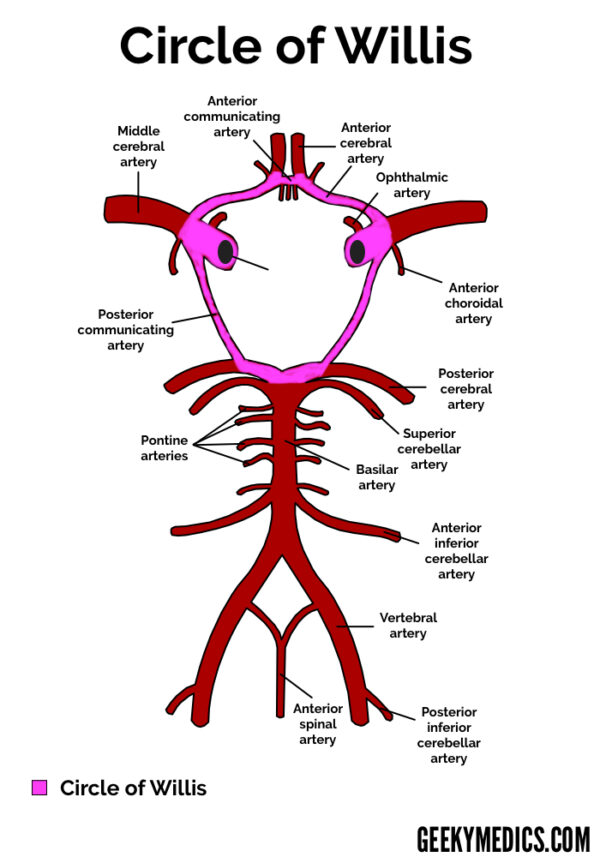
Circle of Willis
Anatomy of the Circle of Willis
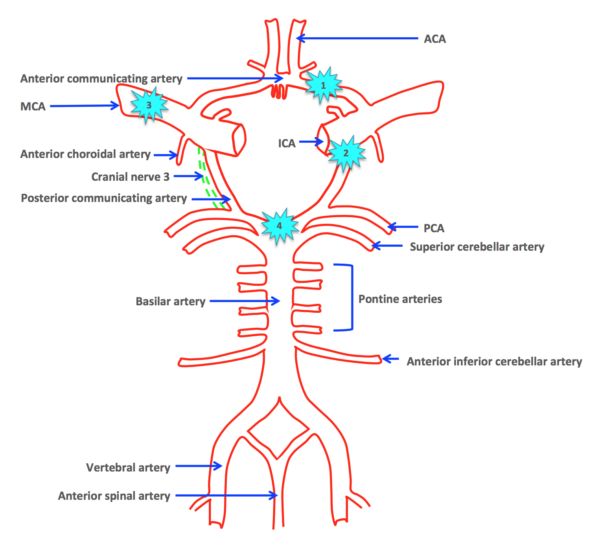
As outlined above, the anterior and posterior circulation terminal branches form an anastomosis to create a ring-like vascular structure known as the circle of Willis, within the base of the cranium (highlighted in pink below).
The left and right internal carotid arteries continue as the middle cerebral arteries (MCA), after each giving off a branch to supply the anterior cerebral arteries (ACA). The anterior communicating artery links the two anterior cerebral arteries together.
The internal carotid arteries also give off the posterior communicating arteries (PCoA), linking the middle cerebral arteries (MCA) with the posterior cerebral arteries (PCA). 8
Clinical relevance: Aneurysms
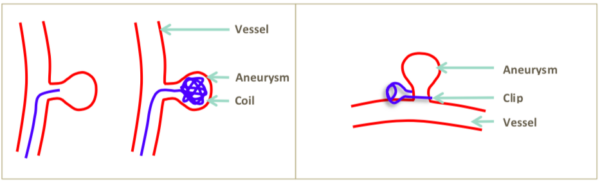
Saccular or ‘berry‘, aneurysms occur most frequently within the circle of Willis vasculature and are the most common cause of non-traumatic subarachnoid haemorrhage. Treatment involves urgent neurosurgical referral and subsequent endovascular coiling or inserting a surgical clip to occlude flow to an aneurysm. 9
Clinical relevance: Third cranial nerve palsy
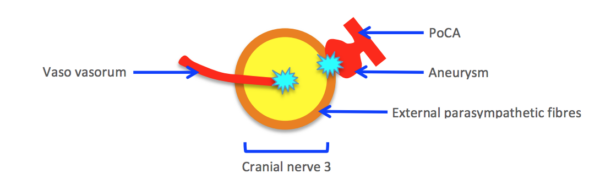
The third cranial nerve is commonly affected by aneurysms in the circle of Willis, particularly those involving the posterior communicating artery (PoCA) due to its close anatomical relationship.
Clinically, “surgical” third nerve palsy can be differentiated from “medical” third nerve palsy by evidence of pupillary involvement. External compression of the third nerve affects parasympathetic fibres surrounding the outermost region of the third nerve. This compression results in an inability to constrict the pupil, making it appear fixed and dilated (often referred to as a ‘blown pupil’).
“Medical” third nerve palsy results from involvement of the vaso vasorum, which is involved in supplying the central area of the third cranial nerve. This results in pupillary involvement arising much later. Common causes of “medical” third nerve palsy include those affecting microvasculature, such as diabetes and atherosclerosis. ²
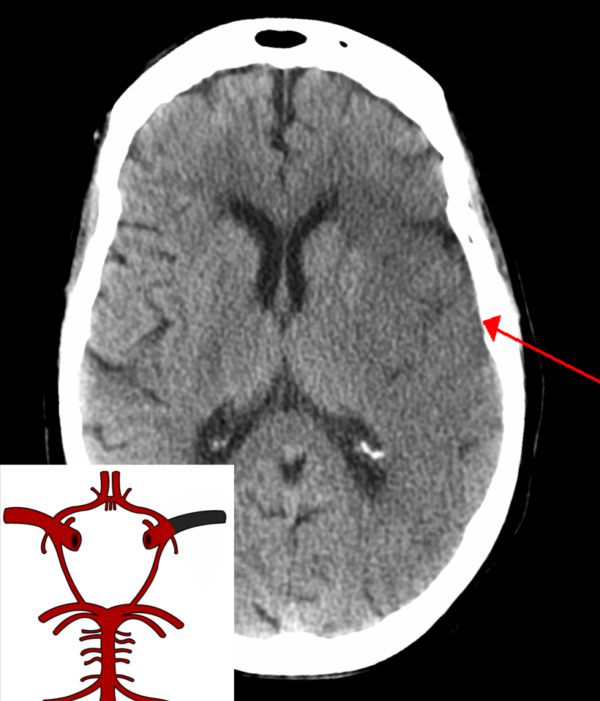
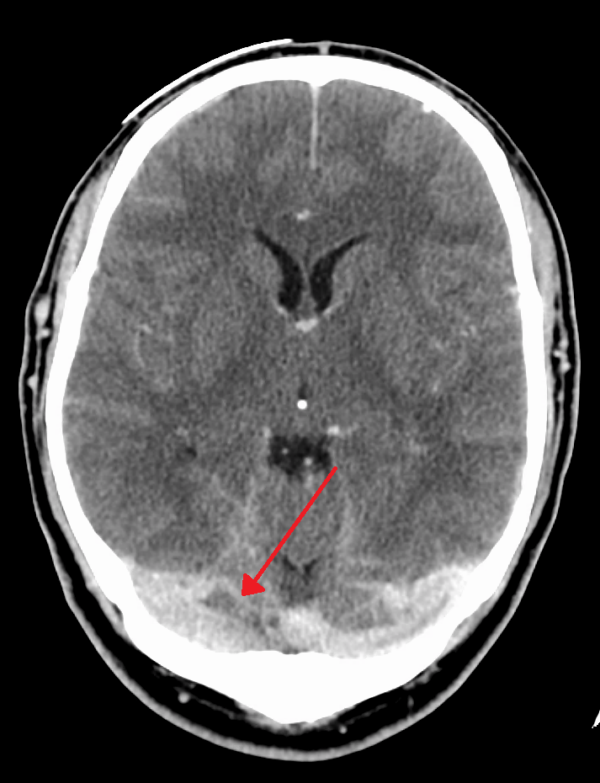
Stroke
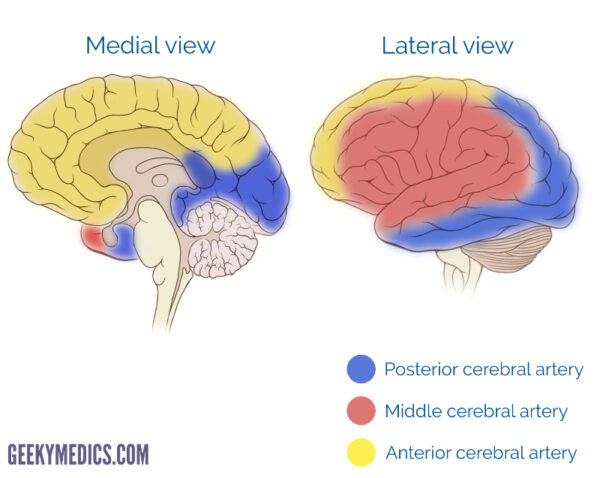
The anterior, middle, and posterior cerebral arteries supply a different territory of the brain (see the image below).
Each region of the brain has specific associated functions, enabling clinicians to discern the site of pathology through history and neurological examination of the patient.
As discussed earlier, the brain is extremely vulnerable to stress in the form of depleted blood supply. A cerebrovascular event (stroke) is a clinical syndrome caused by disruption of blood supply to the brain, characterised by rapidly developing signs of focal or global disturbance of cerebral functions, lasting for more than 24 hours or leading to death.
Stroke types
Strokes can be classified into two major categories: 10
- Ischaemic stroke (87%)
- Haemorrhagic stroke (13%)
Ischaemic stroke
Ischaemic strokes occur when the blood supply to an area of the brain is reduced, resulting in tissue hypoperfusion.
There are several mechanisms which can result in an ischaemic stroke, including:
- Embolism: An embolus from somewhere else in the body (e.g. the heart, commonly secondary to atrial fibrillation) causes obstruction of a cerebral vessel, resulting in hypoperfusion to the area of brain the vessel supplies.
- Thrombosis: A blood clot forms locally within a cerebral vessel (e.g. due to atherosclerotic plaque rupture).
- Systemic hypoperfusion: Reduced blood supply to the entire brain secondary to systemic hypotension (e.g. cardiac arrest).
- Cerebral venous sinus thrombosis: Blood clots form in the veins that drain the brain, resulting in venous congestion and hypoxia which damages brain tissue.
Ischaemic strokes are classified using the Bamford Classification System, which uses clinical signs to determine the territory of the infarct.
Haemorrhagic stroke
Haemorrhagic strokes occur when there is a rupture of a blood vessel or abnormal vascular structure within the brain.
There are two sub-types of haemorrhagic stroke:
- Intracerebral haemorrhage: Bleeding within the brain itself secondary to a ruptured blood vessel.
- Intraparenchymal (bleeding within the brain tissue)
- Intraventricular (bleeding within the ventricles)
- Subarachnoid haemorrhage: Bleeding that occurs outside of the brain tissue, between the pia mater and arachnoid mater.
References
- Brucher, J.M. (1988) ‘Pathophysiology of brain ischaemia’, Acta Neurologica Belgica, 88(1), 19-24.
- Fitzgerland, M.T.J., Gruener, G., Mtui, E. (2012) Clinical Neuroanatomy and Neuroscience. 6 edn. United States: Saunders.
- Van Beek, A.H., Claassen, J.A., Rikkert, M.G. and Jansen, R.W. (2008) ‘Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly’, Journal of Cerebral Blood Flow & Metabolism, 28(6), 1071-85.
- Crossman, A.R., Neary, D. (2014) Neuroanatomy: an Illustrated Colour Text. 5 edn. London: Churchill Livingstone.
- Bouthillier classification of internal carotid artery segments. Available at: https://radiopaedia.org/articles/bouthillier-classification-of-internal-carotid-artery-segments (Accessed: 12th August).
- Cloud, G.C. and Markus, H.S. (2003) ‘Diagnosis and management of vertebral artery stenosis’, Qjm, 96(1), 27-54.
- Locked In Syndrome. Available at: https://rarediseases.org/rare-diseases/locked-in-syndrome/ (Accessed 11th August).
- Alpers, B.J., Berry, R.G. and Paddison, R.M. (1959) ‘Anatomical studies of the circle of Willis in normal brain’, A. M. A. Archives of Neurology & Psychiatry, 81(4), 409-18.
- Van der Schaaf, I., Algra, A., Wermer, M., Molyneux, A., Clarke, M., van Gijn, J. and Rinkel, G. (2005) ‘Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage’, Cochrane Database of Systematic Reviews, (4), CD003085.
- Donnan GA, Fisher M, Macleod M, Davis SM (May 2008). “Stroke”. Lancet. 371 (9624): 1612–23. doi:10.1016/S0140-6736(08)60694-7. PMID 18468545.
Figures
- Figure 1: Melissa Gough, 2018
- Figure 2: Internal Carotid Artery. By OpenStax College [CC BY 3.0], via Wikimedia Commons
- Figure 3: Case courtesy of A.Prof Frank Gaillard, Radiopedia.org
- Figure 4: Grey’s Anatomy. Public domain.
- Figure 5: Case courtesy of A. Prof Frank Gaillard, Radiopedia.org
- Figure 6: By Petit B [CC BY-SA 4.0], from Wikimedia Commons
- Figure 7: Grey’s Anatomy. Public domain,
- Figure 8: Melissa Gough, 2018
- Figure 9: Melissa Gough, 2018
- Figure 10: Melissa Gough, 2018
- Figure 11: Frank Gaillard. Patrick J. Lynch, Medical illustrator (Brain_stem_normal_human.svg) CC BY-SA 3.0
- Figure 12: By CFCF [CC BY-SA 3.0], from Wikimedia Commons
- Figure 13: By James Heilman, MD [CC BY-SA 4.0], from Wikimedia Commons
- Figure 14: By James Heilman, MD [CC BY-SA 3.0], from Wikimedia Commons
- Figure 15: By Lipothymia (Anonymised CT scan from my own practice) CC-BY-SA-3.0 via Wikimedia Commons
Editor
Andrew Gowland