- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Hypertrophic cardiomyopathy is characterised by the presence of an asymmetrical increase in left ventricular wall thickness, not solely explained by abnormal loading conditions (commonly hypertension and aortic stenosis).1
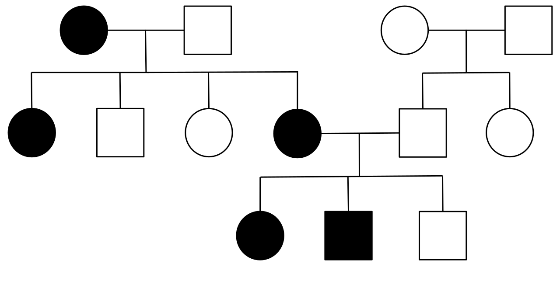
It is typically inherited via autosomal dominant pattern with mutations in cardiac sarcomere protein genes.
Hypertrophic cardiomyopathy has an estimated prevalence of 1 in 500 and most commonly presents in young adults. It is the leading cause of sudden cardiac death in this age group.2
Aetiology
Hypertrophic cardiomyopathy is a primarily genetic condition affecting the sarcomeric proteins. These proteins, namely beta-myosin heavy chain, myosin-binding protein C and cardiac troponin C, are structurally important in cardiac muscle.3
However, the penetrance and expression of responsible genes vary, with complex presentations and sequela. Such diversity in causal mutations and their expression results in an extremely heterogeneous range of phenotypes, each with a different genetic and molecular mechanism responsible for cardiac muscle hypertrophy.
Broadly speaking, hypertrophy is either secondary to functional and structural defects in myocytes or increased storage of materials, such as glycogen.4
These mutations are commonly passed via autosomal dominant inheritance and these patients present with more severe features of the disease.
Disease characteristics
The primary feature of hypertrophic cardiomyopathy is asymmetrical left ventricular wall thickening, typically of the septum. Thickening can be seen in any part of the ventricle.
Other pathological features include myocardial fibrosis/disarray, systolic anterior motion of the mitral valve and abnormal coronary microcirculatory function.
Hypertrophic cardiomyopathy has a bimodal peak occurrence most commonly presenting in the third decade of life.
It is more common in men although the autosomal dominant inheritance pattern is without sex preference. However, hypertrophic cardiomyopathy presents younger in women and they tend to be more symptomatic than men.
Risk factors
The only risk factor for hypertrophic cardiomyopathy (given it is a mostly inherited condition) is family history.
Clinical features
Most affected individuals do not experience substantial symptoms and remain undiagnosed. Consequently, the diagnosis of hypertrophic cardiomyopathy is commonly incidental.6
History
Typical symptoms of hypertrophic cardiomyopathy include:7
- Dyspnoea (90%): due to left ventricular diastolic dysfunction and resultant pulmonary oedema.
- Syncope and presyncope: due to inadequate cardiac output (especially on exertion) or arrhythmias. Patients with syncope are at high risk of sudden cardiac death.
- Chest pain: due to microvascular complications of the disease or mismatch between increased oxygen requirement of the hypertrophied myocardium and reduced perfusion of coronary arteries due to impaired diastolic relaxation.
- Palpitations: due to arrhythmias, both supraventricular and ventricular in origin.
Other important areas to cover in the history include:
- Family history: sudden cardiac death, unexplained heart failure, cardiac transplantation or implantable cardioverter-defibrillator (ICD) insertion.
Construction of a thorough pedigree can identify other family members at risk.
Clinical examination
In patients with suspected hypertrophic cardiomyopathy, a thorough cardiovascular examination is required.
Examination is often unremarkable and findings can be unspecific, however, typical clinical findings in hypertrophic cardiomyopathy include:
- Systolic ejection murmur loudest between the apex and left sternal border: indicative of left ventricular outflow tract obstruction (accentuated with Valsalva manoeuvre).
- Fourth heart sound (S4) of atrial systole against a non-compliant ventricle.
- Holosystolic murmur loudest at the apex or axilla: indicative of mitral regurgitation.
- Double apical beat of ventricular contraction and left atrial contraction against hypertrophic ventricle.
- Lateral displacement of the apical pulse.
- Splitting of the second heart sound.
- Prominent a wave: indicative of reduced right ventricular compliance with massive left ventricular hypertrophy.
Differential diagnoses
It is important to differentiate hypertrophic cardiomyopathy from other causes of left ventricular hypertrophy including hypertension, aortic stenosis, athletic heart, and cardiac amyloidosis.
A detailed history is essential for making this distinction as well as a thorough review of past medical and family history. Imaging, typically echocardiography, is also important for the exclusion of other differential diagnoses.
Other less common differential diagnoses include:
- Metabolic disorders (e.g. Anderson-Fabry disease, Pompe disease)
- Primary mitochondrial disease
- Neuromuscular disease (e.g. Friedreich’s ataxia)
- Malformation syndromes (e.g. Noonan syndrome, LEOPARD syndrome)
Investigations
Bedside investigations
Relevant bedside investigations include:
- Blood pressure: to exclude hypertension.
- Electrocardiography (ECG): left ventricular hypertrophy manifests as increased voltages in precordial leads and non-specific ST-segment and T-wave abnormalities. Deep, narrow Q-waves are common. P-mitrale reflects left atrial dilatation.
- Ambulatory electrocardiography (ECG): forms part of the initial risk assessment. Primary findings of interest include asymptomatic non-sustained ventricular tachycardia (NSVT) and paroxysmal supraventricular arrhythmias (SVT).
Laboratory investigations
While there is no specific laboratory test required to diagnose hypertrophic cardiomyopathy, testing can help to exclude other causes of ventricular dysfunction and potential precipitating factors.
Relevant laboratory investigations include:
- Full blood count: to look for anaemia.
- Urea and electrolytes: for renal dysfunction.
- Liver function tests: for liver dysfunction.
- Thyroid function tests: thyroid disease can exacerbate left ventricular dysfunction.
- NT-proBNP: quantify the degree, if any, of heart failure.
Imaging
Echocardiography is the first-line investigation in diagnosing and managing patients with hypertrophic cardiomyopathy.
Asymmetrical left ventricular wall thickness ≥15mm in one or more left ventricular myocardial segments in diastole, with no other abnormal loading conditions, is suggestive of the diagnosis.1
Modalities of echocardiography include:
- Transthoracic echocardiography (TTE): is used to assess left ventricular wall thickness during diastole; left ventricular outflow tract obstruction (using Doppler mode); valve abnormalities include systolic anterior motion of the mitral valve; left atrial enlargement and diastolic dysfunction. Images should be acquired at rest, during a Valsalva manoeuvre in sitting and semi-supine positions and then standing if no left ventricular outflow tract gradient has been identified.
- Exercise stress echocardiography: indicated in symptomatic patients with no left ventricular outflow tract obstruction identified with Valsalva manoeuvre.
- Transoesophageal echocardiography (TOE): indicated if images of left ventricular outflow tract mechanism or valve abnormalities are poorly visualised. Perioperative TOE is used to guide surgical strategy prior to septal myomectomy and for detection of surgical complications.
Other relevant imaging investigations include:
- Cardiac magnetic resonance imaging (cMRI): should be considered as part of a thorough assessment if local resources and expertise permit, however, it is not required for diagnosis. cMRI allows superior identification of left ventricular apical and anterolateral hypertrophy and is, therefore, more likely to pick up apical hypertrophic cardiomyopathy as well as other subtle markers of disease.
- Cardiac computed tomography (cCT): consider in patients with poor echocardiography images and contraindications to magnetic resonance imaging.
Specialist tests
Other specialist tests include:
- Cardiopulmonary exercise testing: recommended in all patients and provides functional information about the patient’s cardiac function. Useful in differentiation between athletic hypertrophy and hypertrophic cardiomyopathy.
- Electrophysiological testing: indicated if proven persistent or recurrent supraventricular tachycardia or in patients with ventricular pre-excitation.
Management
General lifestyle advice for all patients includes avoiding dehydration and alcohol, encouraging weight loss and safety netting for symptoms of deterioration.
Angina
Beta-blockers and calcium channel blockers can be used in patients with prolonged or exertional angina-like chest pain in the absence of significant left ventricular outflow tract obstruction and coronary artery disease.
Safe use of oral nitrates relies on the exclusion of left ventricular outflow tract obstruction and, if used, should be done so cautiously.1
Atrial fibrillation
The prevalence of atrial fibrillation in patients with hypertrophic cardiomyopathy is 22.5%.1
The two greatest predictors for paroxysmal or permanent atrial fibrillation are age and left atrial enlargement. If left atrial diameter is ≥45mm, the patient should undergo 6 to 12 monthly, 48-hour ambulatory electrocardiogram monitoring to exclude atrial fibrillation.8
Management of atrial fibrillation depends on the haemodynamic status of the patient:
- Haemodynamically unstable: emergency direct current cardioversion
- Haemodynamically stable: beta-blockers or verapamil/diltiazem
Some important considerations for rate and rhythm control of atrial fibrillation in hypertrophic cardiomyopathy:
- Digoxin is not suitable in patients with left ventricular outflow tract obstruction and a normal ejection fraction
- Flecainide should be avoided as it may prolong the QT interval
Anticoagulation is recommended in all patients with atrial fibrillation given their younger age and elevated risk of thromboembolic events. Continuation of lifelong anticoagulation, despite cardioversion and maintenance of sinus rhythm, is also recommended.
CHA2DS2-VASc scoring is not appropriate. HAS-BLED scoring can be used to evaluate a patient’s risk of bleeding.8
Warfarin is the anticoagulant of choice. There is currently no data on the use of novel oral anticoagulants, and these, therefore, are only indicated if warfarin is not tolerated.
Heart failure
Management of heart failure in patients with hypertrophic cardiomyopathy is divided depending on the patients’ ejection fraction. In severe cases, cardiac resynchronisation therapy and cardiac transplant may be considered:
- Preserved ejection fraction: reduce left ventricular diastolic pressure and improve left ventricular filling with beta-blockers, verapamil or diltiazem and, if indicated, loop diuretics.
- Reduced ejection fraction: a threshold ejection fraction of <50%, given the preservation of cavity size in patients with hypertrophic cardiomyopathy, is recommended when considering renin-angiotensin-aldosterone system inhibition.9 Below this threshold, management with diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) and mineralocorticoid receptor antagonists (MRA) is recommended in keeping with heart failure guidelines.
Cardiac transplantation can be considered in patients with NYHA functional class III or IV symptoms, refractory to medical management and without left ventricular outflow tract obstruction.
Implantable cardioverter-defibrillator (ICD)
An implantable cardioverter-defibrillator is indicated as secondary prevention in survivors of a cardiac arrest due to ventricular tachycardia or ventricular fibrillation and patients with sustained ventricular tachycardia causing syncope or haemodynamic instability.
Primary prevention with an implantable device is considered within a clinical context alongside risk factors for sudden cardiac death.1
Left ventricular outflow tract obstruction (LVOTO)
Left ventricular outflow tract obstruction (LVOTO) is defined by tract pressure gradients ≥30mmHg. Gradient ≥50mmHg is the threshold at which left ventricular outflow tract obstruction becomes haemodynamically significant.
Investigation of LVOTO is initially with 2D and Doppler echocardiography at rest, Valsalva and on standing. Further management depends on the peak provoked tract gradient.
If on exercise stress echocardiography the outflow tract gradient is >50mmHg then septal reduction therapy is indicated. If <50mmHg, medical therapy is the mainstay of treatment.10
The purpose of medical therapy is to improve exercise tolerance, improve symptoms and improve left ventricular diastolic filling.
Medications include vasodilating beta-blockers initially, followed by disopyramide, if QTc within normal limits, or verapamil, if beta-blockers are ineffective or contraindicated.1 Low-dose loop diuretics may improve dyspnoea.
If symptoms of left ventricular outflow tract obstruction remain despite best medical therapy, septal reduction therapy can be considered. The two main procedures are ventricular septal myomectomy or septal alcohol ablation.
Indications for septal reduction therapy are:
- Left ventricular outflow tract gradient ≥50mmHg
- New York Heart Association (NYHA) grade III or IV
- Recurrent exertional syncope despite medical treatment
If myomectomy and septal alcohol ablation are contraindicated, dual-chamber, biventricular pacing has been shown to improve quality of life and functional capacity in selected patients.12
Follow-up
All patients with unexplained hypertrophic cardiomyopathy should receive genetic counselling including testing of first-degree relatives.
Patients should have an annual follow-up with ambulatory ECG monitoring.
Complications
Complications of hypertrophic cardiomyopathy include:13
- Sudden cardiac death
- Arrhythmias: supraventricular (especially atrial fibrillation) and ventricular in origin
- Heart failure
- Infective endocarditis
Risk factors for sudden death
Although the annual rate of sudden cardiac death in patients with hypertrophic cardiomyopathy is estimated at less than 1%, some specific groups remain at much higher risk.14
The risk for sudden cardiac death can be estimated based on the following factors:1
- Maximal left ventricular wall thickness
- Left atrial diameter
- Maximal left ventricular outflow tract gradient
- Family history of sudden cardiac death
- Non-sustained ventricular tachycardia
- Unexplained syncope
- Age
HCM Risk-SCD is a validated prediction tool used for sudden cardiac death risk in hypertrophic cardiomyopathy.
Key points
- Hypertrophic cardiomyopathy is unexplained, usually asymmetrical, thickening of the left ventricular wall.
- It is commonly inherited via autosomal dominant trait.
- Typical symptoms include shortness of breath, chest pain, palpitations, pre-syncope and syncope.
- Clinical examination is often normal. However, identification of an ejection systolic murmur can indicate left ventricular outflow tract obstruction.
- Important investigations include electrocardiography, both 12-lead and ambulatory, and diagnostic imaging.
- A left ventricular wall thickness of ≥15mm during diastole is diagnostic in any imaging modality. The first choice is echocardiography.
- Management is initially medical with vasodilating beta-blockers in the first instance. Septal reduction therapy may be considered if medical therapy fails and left ventricular outflow tract obstruction has been identified.
- Septal reduction therapies include surgical myomectomy or septal alcohol ablation.
- Implantable cardioverter-defibrillators (ICDs) are indicated following arrhythmias of ventricular origin with haemodynamic compromise or following a careful risk assessment.
- Sudden cardiac death, arrhythmias and heart failure are common complications of hypertrophic cardiomyopathy.
Reviewer
Dr Chih Wong
Consultant Cardiologist
Editor
Dr Chris Jefferies
References
- European Society of Cardiology. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Published August 2014. Available from: [LINK]
- Circulation. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Published August 1995. Available from: [LINK]
- National Library of Medicine (US). Genetics Home Reference. Familial Hypertrophic Cardiomyopathy. Published May 2020. Available from: [LINK]
- Circulation Research. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Published September 2017. Available from: [LINK]
- Figure 1. Wikimedia. A typical pedigree for autosomal dominant inheritance. License: [CC-BY-SA]. Available from: [LINK]
- The Lancet. Hypertrophic cardiomyopathy. Published August 2012. Available from: [LINK]
- Medscape. Hypertrophic Cardiomyopathy Clinical Presentation. Published January 2016. Available from: [LINK]
- Arrhythmia and Electrophysiology Review. Atrial fibrillation and anticoagulation in hypertrophic cardiomyopathy. Published April 2017. Available from: [LINK]
- JACC: Heart Failure. Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. Published May 2018. Available from: [LINK]
- European Society of Cardiology. Investigation of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Available from: [LINK]
- American Heart Journal. Therapeutic effect of oral dipyridamole on myocardial perfusion and cardiac performance in patients with hypertrophic cardiomyopathy. Published February 1992. Available from [LINK]
- Global Cardiology Science and Practice. Cardiac pacing in patients with hypertrophic obstructive cardiomyopathy. Published August 2018. Available from: [LINK]
- Patient.info. Hypertrophic Cardiomyopathy. Published August 2017. Available from: [LINK]
- European Society of Cardiology. Prevention of sudden death in hypertrophic cardiomyopathy: bridging the gaps in knowledge. Published July 2016. Available from: [LINK]




