- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Immunology seems to be one of those things that people either love or hate; I think it’s fascinating, but I know there will be a lot of people out there who approach the subject with a mixture of terror, frustration and loathing. To make the normal immune response less of a horrendous nightmare to learn, I made a summary diagram showing friendly, loveable cartoon immune cells doing what they do best – pwning pathogens. I know it looks pretty complicated, but don’t panic! I’m also going to explain everything step by step, so by the end of this article it will all make sense and you’ll feel super clever.
You can click on the diagram to enlarge it, and please feel free to download and print it too.
P.S. This article is a bit of a whopper, so while things are printing I would nip and get a cup of tea/coffee/your unhealthy revision fuel of choice to ensure you’re sufficiently energised before you start!
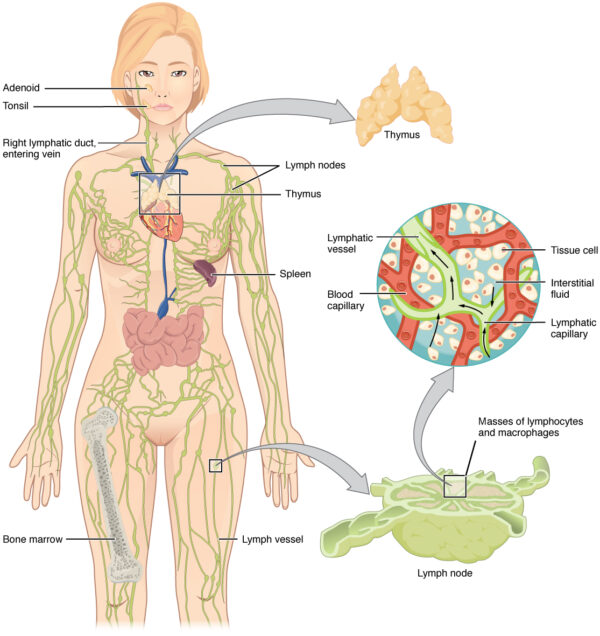
Anatomy of the immune system
The immune system is a mobile, circulating system. However, there are some fixed anatomical structures that are important to its function:
- immune cells are made within the bone marrow during haematopoiesis
- the thymus gland is situated just in front of the heart in the mediastinum. It is active throughout life, but is at its largest in childhood and decreases in size after puberty. It is where lymphocytes mature and receive their immunological “education” before being released into the bloodstream.
- mature lymphocytes migrate to lymph nodes, which are small bean-like structures situated along the lymphatic vasculature throughout the body. These filter lymph and provide a site for antigen presentation to the adaptive immune system. Lymph is then returned to the systemic circulation via the thoracic duct, which joins with the left subclavian vein.
- the spleen is basically a massive lymph node and is, therefore, another site of antigen presentation to mature lymphocytes. It is part of the reticulo-endothelial system which filters blood and removes old cells, tissue debris, pathogens and immune complexes. It also stores red blood cells and immature monocytes.
- finally, the liver is also a site of antigen presentation and contains its own cohort of phagocytes and lymphocytes. This is a vital role, as the liver filters large volumes of potentially contaminated venous blood from the gastrointestinal (GI) tract. It also synthesises acute phase proteins such as CRP in response to infection.
Barrier mechanisms of the immune system
There are numerous potential ways for pathogens to enter the body. Humans have therefore evolved several physical and chemical barrier mechanisms to prevent the invasion of infective organisms:
- intrinsic epithelial barriers exist between the body and the outside world. Epithelial cell walls have very tight junctions between them and are therefore hard to penetrate. Examples include the linings of the mouth, nasal passages, upper airways, lungs and GI tract.
- the continuous longitudinal flow of air or fluid through most body systems helps to create a flushing action which prevents situations in which bacteria could adhere to structures, proliferate and invade
- the movement of mucus by cilia in the lungs also helps prevent the stagnation of secretions and the adherence of inhaled droplets and particles. Mucus is moved upwards towards the pharynx, where it is then swallowed or coughed up.
- desquamation of skin and epithelial cells also prevents adherence of microorganisms
- natural acids persist in many parts of the body, for example, fatty acids on the skin, lysozymes in saliva and hydrochloric acid in the stomach
- there are also many natural antibacterial peptides on the skin and the surface linings of the lungs and gut. These include cathelicidins, defensins, proteinase inhibitors and chemokines.
- normal bacterial flora colonising various parts of the body compete with infective microorganisms, and some also produce antimicrobial substances. For example, vaginal lactobacilli produce lactate, which creates an acidic environment and destroys many potentially infectious organisms.
Cells of the immune system
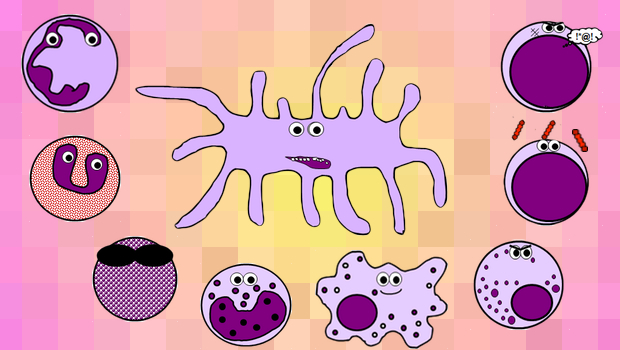
There are many different groups of cells involved in the immune response. Depending on which medical school you’re at, you may be expected to be able to recognise them on microscopy, so I’ve included some pictures of actual real cells alongside my silly cartoon ones.
GRANULOCYTES
A family of white blood cells containing granules in their cytoplasm
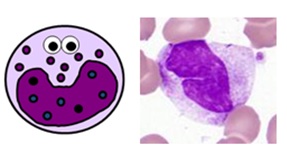
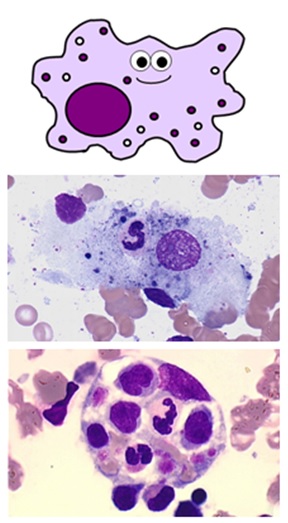
NEUTROPHILS
- normally make up 40-75% of all white blood cells (2-7.5 x 109/L on a full blood count)
- the first line of defence against all infections
- act by phagocytosing invading organisms and presenting antigens to the immune system
- they have segmented nuclei and their cytoplasm is full of pinky-purple intracellular granules

Neutrophil
EOSINOPHILS
- normally make up 1-6% of white blood cells (0.04-0.44 x 109/L on a full blood count)
- they specifically act against multicellular parasites (e.g. worms) by dissolving their cell surfaces
- they are also involved in IgE-mediated allergic disorders such as asthma
- they have bilobed nuclei and intracellular granules which stain brick red with eosin

Eosinophil
BASOPHILS
- normally make up 0-1% of white blood cells (≤0.01 x 109/L on a full blood count)
- they are the circulating counterparts of tissue mast cells and are rather mysterious
- they probably have roles in inflammation, parasitic infections and allergic reactions
- mast cells/basophils have an important role in type 1 hypersensitivity reactions through their binding with IgE antibodies
- they have bilobed nuclei and large darkly staining intracellular granules
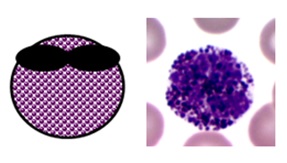
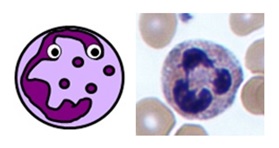
MONOCYTES & MACROPHAGES
Large cells involved in phagocytosis and antigen presentation
BLOOD MONOCYTES
- normally make up 2-10% of white blood cells (0.2-0.8 x 109/L on a full blood count)
- they are produced in the bone marrow and travel in the bloodstream to their target tissues, where they become macrophages
- they have roles in phagocytosis, antigen presentation and cytokine production
- they are large cells with fine “ground-glass” granules and horseshoe-shaped nuclei

Monocyte
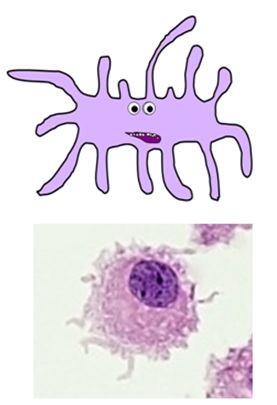
TISSUE MACROPHAGES
- these are tissue cells and therefore aren’t found on a full blood count
- they are derived from blood monocytes, which differentiate once they reach their target tissues and express CD14 receptors
- they “tidy up” any pathogens, foreign debris and old or dead cells from their tissues using phagocytosis
- they also perform antigen presentation and can activate memory cells
- like monocytes, they are large cells with horseshoe-shaped nuclei
- the name macrophage means “big eater”. As you can see, they can be quite difficult to identify depending on how much they’ve eaten!
- they have processes on their cell membranes called pseudopodia which extend around the unlucky item they’re about to eat
- once internalised, the engulfed material is contained within a large vesicle called a phagosome
- the phagosome is fused with another vesicle called a lysosome containing either reactive oxygen species or enzymes, which break down its contents
- there are many types of macrophage which are specifically adapted to different tissues – these include Küpffer cells in the liver, alveolar macrophages in the lungs, osteoclasts in bone and microglial cells in neurones

Macrophages
DENDRITIC CELLS
- this complex family of cell types are the main “professional” antigen-presenting cells of the immune system
- they play a vital role in activating T helper cells and memory cells
- they are formed in the bone marrow and circulate in the bloodstream until they reach their target tissues, where they are activated by pathogens and differentiate into their mature forms
- they phagocytose pathogens before migrating to lymph nodes, where they present antigens on their cell surfaces with the costimulatory molecules required to activate the adaptive immune response
- they have numerous characteristic “dendritic” processes branching from their cell membranes
- there are several specialised dendritic cell types, including Langerhans cells in the skin

Dendritic cells
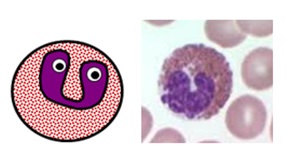
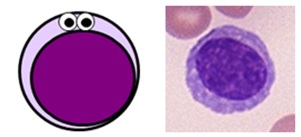
LYMPHOCYTES
Small, specialised white blood cells with large nuclei and no granules
- normally make up 20-45% of all white blood cells (1.3-3.5 x 109/L on a full blood count)
- there are three main subtypes of lymphocytes: B cells, T cells and natural killer (NK) cells
- B cells and T cells make up the majority of the lymphocyte population. They are small cells with large round nuclei, scanty blue-ish cytoplasm and no granules, and are morphologically indistinguishable from one another. The only way to tell them apart is with specialist serology or staining for specific cell surface markers known as clusters of differentiation (CDs).
- NK cells are a larger, more primitive lymphocyte subtype which do contain some granules.

Lymphocyte
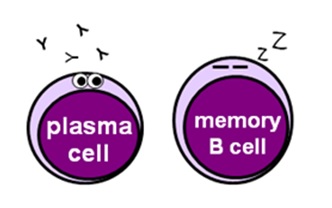
B CELLS
- B cells represent about 25% of the total lymphocyte population – this varies depending on the activity of the immune response and can be up to 50%
- important B cell surface markers include CD19, CD20 and CD21, as well as MHC II
- they are essential for humoral immunity, also known as the antibody-mediated immune response
- plasma cells are mature B cells that secrete antibodies, which recognise specific foreign antigens and bind to them or destroy them
- memory B cells “remember” the offending foreign antigens to allow the immune system to mount a quicker antibody response to any subsequent infections
T CELLS
- T cells represent about 70% of the total lymphocyte population – this varies depending on the activity of the immune response and can be up to 90%
- all T cells express CD3 on their surfaces, along with T cell receptors (TCRs) which recognise specific antigens presented in an MHC I or MHC II molecule
- there are numerous different T cell subtypes with different roles, which each have their own identifiable surface markers – there are absolutely loads of these, so I’ve only discussed the most important ones below
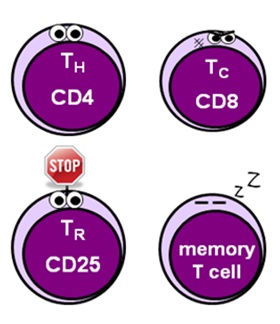
- helper T cells (CD4) facilitate the activation of the immune response and stimulate division and differentiation of various effector cells
- cytotoxic T cells (CD8) – also known as killer or effector T cells – provide cell-mediated immunity by targeting and killing infected cells
- regulatory T cells (CD25 + FOXP3) – also known as suppressor T cells – play a vital role in limiting the immune response to prevent excessive damage to tissues and organs
- memory T cells (CD62 + CCR7) “remember” what has happened to allow the immune system to mount a faster, more effective response should the offending organism be foolish enough to return
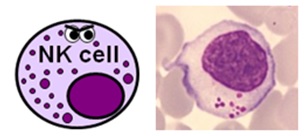
NATURAL KILLER CELLS
- NK cells represent about 5% of the total lymphocyte population – again, this varies depending on what’s going on
- they are a larger, primitive lymphocyte subtype with granules in their cytoplasm – they are also known by haematologists as large granular lymphocytes (LGLs)
- they express CD16 and CD56, and a large proportion of them also express CD8
- NK cells actually form part of both the innate and adaptive immune systems and are able to destroy pathogens and infected cells without the need for prior activation by specific antigens.
- They are also particularly important in viral immunity and tumour rejection.

Natural killer cells
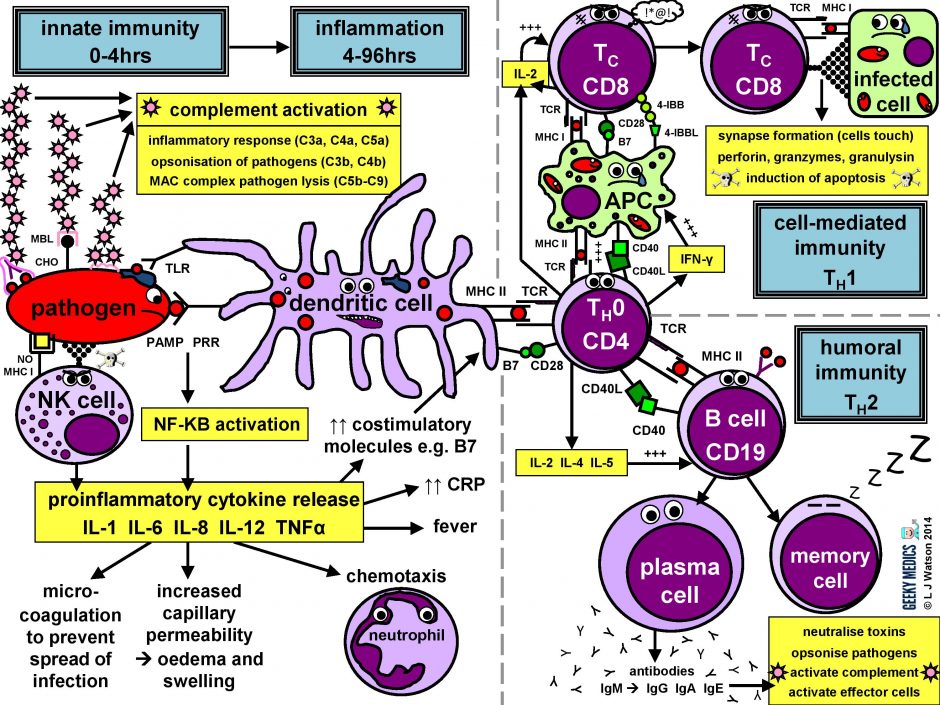
The immune response in a nutshell
The normal immune response can be broken down into four main components:
- pathogen recognition by cells of the innate immune system, with cytokine release, complement activation and phagocytosis of antigens
- the innate immune system triggers an acute inflammatory response to contain the infection
- meanwhile, antigen presentation takes place with the activation of specific T helper cells
- CD4 helper T cells then co-ordinate a targeted antigen-specific immune response involving two adaptive cell systems: humoral immunity from B cells and antibodies, and cell-mediated immunity from cytotoxic CD8 T cells
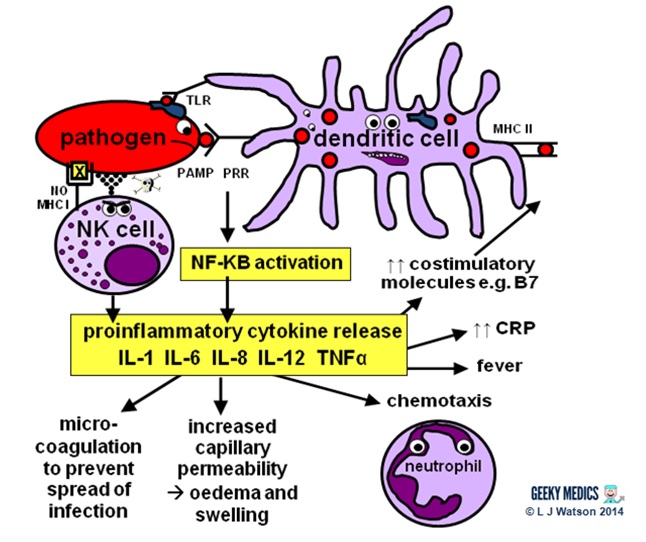
Let’s start at the very beginning. An evil pathogen has penetrated the body’s barrier mechanisms and is plotting to start a nasty infection…
Part 1 – Innate immune system
This is the first line of defence against any infection. It is very fast – it is established within about 4 hours – but is non-specific and has no memory, so it is not strong enough to effectively tackle an infection on its own. It consists of a cellular response by the innate immune system, a chemical response by cytokines and complement, and the subsequent initiation of an acute inflammatory response.
INNATE CELLULAR IMMUNE RESPONSE
Phagocytes
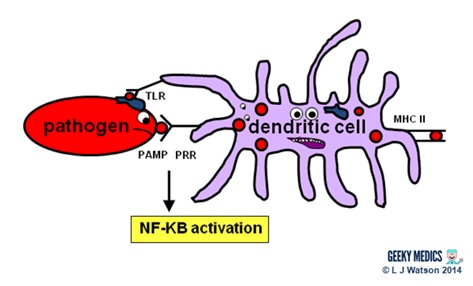
Phagocytes are a very important part of the innate immune system, as they act to fight the new infection and present antigens. Examples of “professional” phagocytes include dendritic cells, blood monocytes, tissue macrophages and, most importantly, neutrophils. Neutrophils are an absolutely key component of this initial process which only appear in response to infection or injury, and are therefore not found in healthy tissue.
- phagocytes identify pathogens by recognising pathogen-associated molecular patterns (PAMPs) using pathogen recognition receptors (PRRs). Toll-like receptors (TLRs) are an example of a PRR.
- once they have identified dangerous organisms, they internalise them, kill them and digest them down into their component proteins
- phagocytes then present the digested protein antigens to the cells of the adaptive immune system via major histocompatibility complexes (MHCs) on their surfaces. The MHC complex acts as a safety mechanism. It prevents the immune system from being activated too easily, as it ensures that T cells can only react to an antigen if it is presented within an MHC complex. This phenomenon is known as MHC restriction.
- when phagocyte PRRs are exposed to PAMPs, NFKB is activated. This is a transcription factor which results in the release of proinflammatory cytokines and the initiation of the inflammatory response.
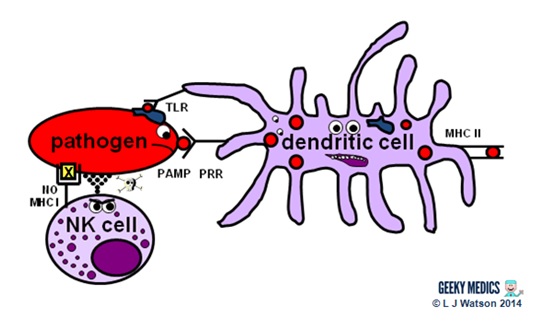
Natural killer cells (NK cells)
Natural killer cells, along with neutrophils and other phagocytes, also have an important role in the initial front-line defence against the infection.
- unlike T cells, they do not require activation by specific antigens, which means they are able to respond immediately when exposed to a pathogen
- “self” cells are protected from the destructive action of NK cells by the inhibitory effects of MHC I, which is expressed on the surface of all nucleated body cells
- any cells without an identifiable MHC I are likely to be “non-self” and fair game for immediate annihilation – NK cells do this by releasing toxic granules to induce apoptosis
- normally, NK cells cannot attack healthy “self” cells. However, MHC I expression is often suppressed if cells are infected with viruses, or have become cancerous. NK cells can, therefore, perform additional vital roles in viral immunity and tumour rejection.
INNATE CHEMICAL IMMUNE RESPONSE
Complement system
Complement is a cascade of chemicals similar to the clotting cascade.
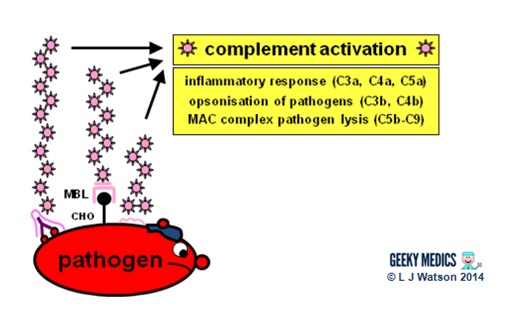
There are three separate pathways that activate the complement system:
- classical pathway: activated by antibody-antigen complexes (a.k.a immune complexes) on pathogen surfaces
- mannose-binding lectin pathway: activated when mannose-binding lectin binds to the carbohydrate molecule mannose on pathogen surfaces
- alternative pathway: C3 reacts directly with pathogen surfaces
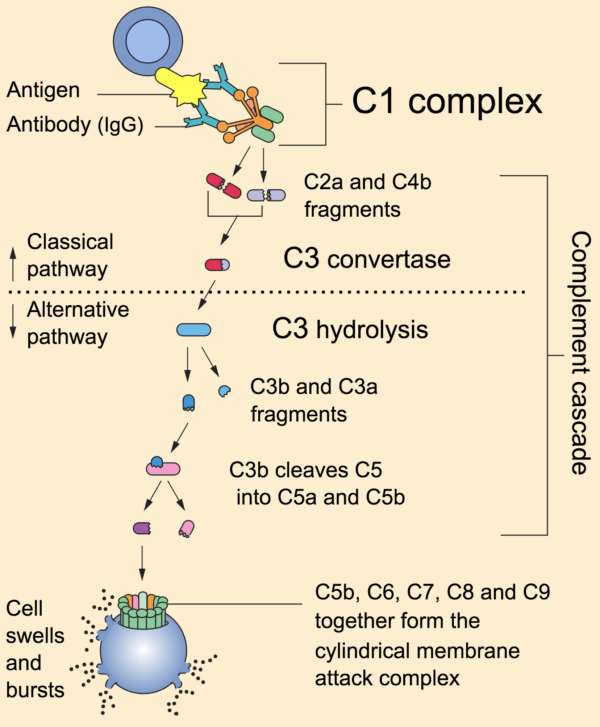
All three of these pathways act to generate the enzyme C3 convertase. This cleaves C3 into two parts (C3a and C3b) and activates the rest of the cascade.
- C3a, along with C4a and C5a, is a mediator of inflammation which augments the inflammatory response. These molecules are also anaphylotoxins which trigger mast cell degranulation, histamine release and further inflammation.
- C3b binds to and coats pathogens, making them easier for phagocytes to identify and ingest. This process is called opsonisation. It also binds to immune complexes to facilitate their removal by the spleen and triggers the production of terminal components including C5b.
- C5b initiates the membrane attack pathway or “terminal lytic sequence”. This triggers the formation of a membrane attack complex (MAC) made from C5b, C6, C7, C8 and C9. MACs are ring-shaped and essentially punch a hole in the pathogen cell membrane, resulting in osmotic lysis.
- complement mainly provides bacterial immunity. In viral infections, interferons play a similar role.
Proinflammatory cytokines are the second key component of the innate chemical immune response. They are small messenger proteins released by immune cells in response to evidence of infection, which interact to mediate the acute inflammatory response (see Part 2). There are a huge number of different cytokine molecules, including whole families of interleukins, tumour necrosis factors and chemokines.
Some important examples include:
- IL-1 – causes fever and activates lymphocytes
- IL-6 – causes fever, stimulates the liver to produce acute-phase proteins such as CRP, activates lymphocytes and promotes antibody production
- IL-8 (a.k.a CXCL8) – causes neutrophil chemotaxis
- IL-12 – activates NK cells and TH1 cells (important for intracellular infections)
- TNF-alpha – increases vascular permeability to allow immune cells to reach tissues
- IL-4, IL-5 + IL-13 – promote IgE production and eosinophilic reactions in patients with allergies
- Interferon gamma (IFNγ) – essential in activating cell-mediated immunity in viral infections
- IL-10 – has an anti-inflammatory effect
Part 2 – Inflammatory response
The acute inflammatory response is kick-started by innate immune cells, proinflammatory cytokines and complement. It acts as a bridging mechanism to localise and contain the infection in the period from about 4-96 hours after its onset, when the innate immune system is running out of steam and the specific cellular immune response is still trying to get going.
The main features of this process are:
- vasodilation and increased blood flow – this leads to erythema (“rubor”) and warmth (“calor”)
- increased vascular permeability – this allows an inflammatory cell infiltrate to extravasate and reach the site of infection, and also causes tissue oedema and swelling (“tumour”)
- release of inflammatory mediators such as bradykinins and prostaglandins which increase pain sensitivity and cause hyperalgesia in the infected area (“dolor”)
- neutrophil chemotaxis – neutrophils migrate to the site of infection and begin their clean-up operation, phagocytosing pathogens and debris
- microvascular coagulation – this is induced by local tissue damage, and acts to confine the infection and prevent its spread
- systemic features such as fever and raised inflammatory markers such as CRP and ferritin – this produces unpleasant “flu-like” symptoms such as hot flushes, sweats, chills, rigors, headache, nausea, myalgia, arthralgia and fatigue.
- upregulation of costimulatory molecules such as MHC-II and B7 to encourage activation of the adaptive immune system
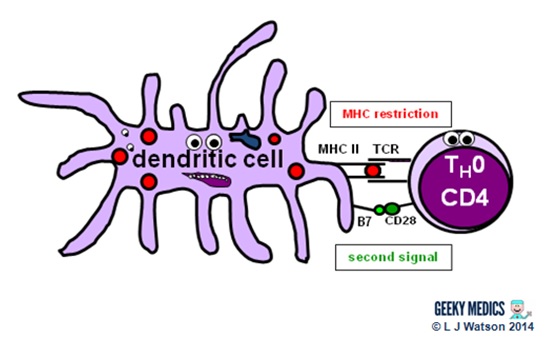
Part 3 – Antigen presentation
The innate immune system and inflammatory response can only hold off an infection for so long – ultimately, a specific immune response needs to be activated. This is done via antigen presentation to the adaptive immune system.
- dendritic cells laden with digested antigens travel via the circulation to lymph nodes
- once they arrive there, they start to present their antigens to naive T helper cells (TH0) within MHC II complexes on their cell surfaces
It is very important that the immune response is not activated inappropriately, as this could cause a lot of unnecessary damage. There are two main protective mechanisms which prevent this from happening by controlling the activation of the adaptive immune system:
- MHC restriction ensures that only antigens presented within the context of MHC complexes are able to trigger the immune response
- in order to become fully activated by their specific antigen, naive T helper cells also require a second signal from antigen-presenting cells. Dendritic cells are able to provide this in the form of B7 proteins (CD80 or CD86) which bind to CD28 receptors on T cell surfaces.
- expression of second signal molecules is increased by the presence of an inflammatory response, increasing the likelihood of T helper cell activation
The combination of the right antigen, an MHC II and a B7 second signal gives the green light for naive T helper cells to get going. The next step is for them to differentiate into either TH1 cells, which promote cytotoxic T cells and cell-mediated immunity, or TH2 cells, which promote B cells and humoral immunity.
Part 4a – Humoral immunity
Humoral immunity is the term for a specific adaptive immune response activated by TH2 cells, which leads to the production of B cells and antibodies.
This immune response is designed to fight extracellular infections, including most bacteria and fungi, protozoans such as Giardia, and parasitic worms such as Schistosoma.
ANTIBODIES
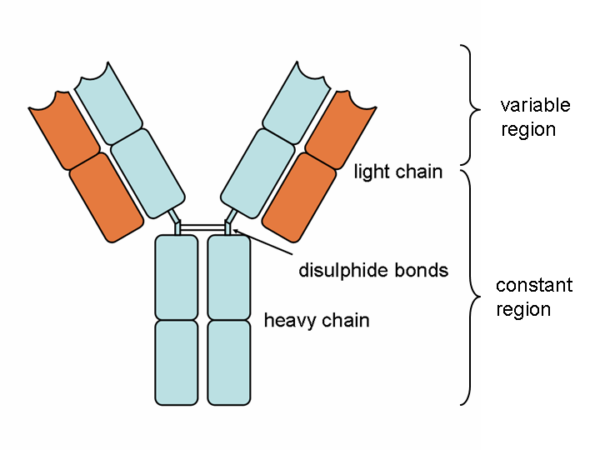
Antibody molecules are essentially secreted B cell receptors which provide an antigen-specific action. They are Y-shaped molecules with a complex structure comprising:
- two large heavy chains – their structure dictates whether the antibody is IgM, IgG, IgA, IgE or IgD
- two small light chains – these can be either “kappa” or “lambda” types
- the heavy and light chains are connected by disulphide bonds
- all four chains consist of constant and variable regions: the constant “C” regions are always the same, but the variable “V” regions are unique to each B cell and confer antigen specificity
- antigens bind to the ends of each “arm” of the Y structure
- the base of the antibody binds to complement and phagocytes
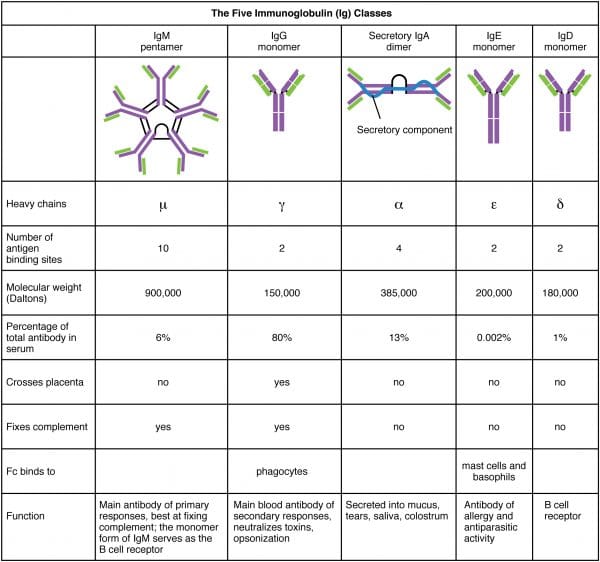
There are five antibody classes or “isotypes”. These are dictated by the structure of the heavy chain constant region.
- IgM – this has a pentameric structure. It is expressed on B cell surfaces and produced early in the immune response whilst IgG is being generated.
- IgG – this has a monomeric structure and provides the majority of antibody-based immunity. It is found mainly in circulating blood and tissues (it also crosses the placenta to provide passive immunity to the fetus).
- IgA – this forms a dimeric structure once it reaches its target tissues. It is found in mucosal areas such as the GI, respiratory and urinary tracts. It is also secreted in saliva, tears and breast milk.
- IgE – this has a monomeric structure. It binds to allergens and mediates allergic reactions, as well as providing immunity against multicellular organisms such as parasitic worms.
- IgD – this has a monomeric structure and is rather mysterious. It is found in very low levels in the serum, and appears to interact with basophils and mast cells.
There are a potentially infinite number of antigens the immune system might encounter, so it is vital that the immune response is able to adapt to this and reflect a similar degree of diversity. Humans need to be able to generate about 10 billion different antibodies, but it would be impossible to code every possible antibody into the human genome and still be able to fit our DNA into such tiny spaces within cells. As a result, a range of mechanisms have evolved to allow B cells to manipulate their own DNA and produce billions of different variable region structures:
- antibody variable region genes are coded in three parts: V (variable), D (diversity) and J (joining) segments. RAG proteins allow B cells to shuffle these gene segments around during their maturation and recombine them in millions of different ways. This is known as VDJ recombination.
- junctional diversity is produced by the imprecise joining of VDJ segments during maturation, as the variable overlap of genes results in the gain or loss of a few nucleotides
- “looping out” and rejoining of gene segments produces variations in genomic structure
- genetic diversity is also increased by the addition of random nucleotides called N regions
- when mature B cells are activated by their specific antigen, they start to produce IgM antibodies, and also undergo isotype class switching to produce different types of antibody adapted for various locations within the body
- B cell activation also promotes somatic hypermutation of variable region genes to produce ever-so-slightly different versions of the same specific antibody. These are “tested” to find the best match using clonal selection, and the ones with the highest possible affinity for the antigen are encouraged to proliferate in a process called affinity maturation.
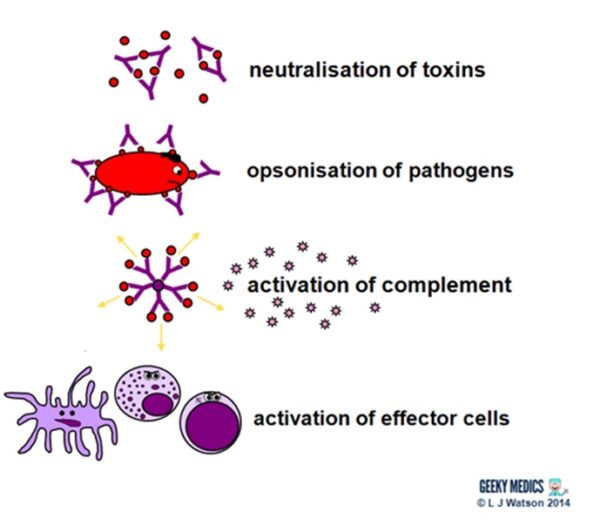
Antibodies fight extracellular infections in a number of ways:
- they neutralise toxins by directly binding to them
- they bind to antigens on pathogen surfaces. This agglutinates them to impair their mobility and also opsonises them to enhance phagocytosis.
- the binding of antibodies to antigens to form complexes activates the classical complement pathway
- they also directly activate effector cells such as dendritic cells, NK cells and cytotoxic T cells
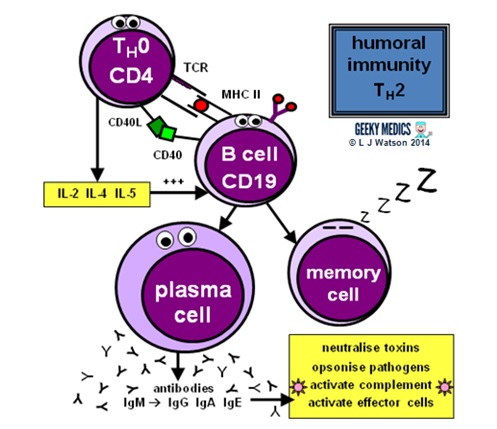
THE HUMORAL IMMUNE RESPONSE
Humoral immunity and antibody production are dependent upon T helper cells activating B cells:
- once naive TH0 cells have been activated by their specific antigen, they differentiate into TH2 cells
- TH2 cells locate their corresponding B cell counterparts by identifying the correct antigen within an MHC II on the B cell’s surface
- they then provide the B cell with a second signal, in this case, CD40 ligand which binds to CD40 on the B cell surface
- they also release cytokines such as IL-2, IL-4 and IL-5 which promote B cell development
Activated B cells mature into plasma cells and start to make antibodies:
- initially, plasma cells produce IgM antibodies, then isotype class switching produces different types to cover different areas of the body
- clonal expansion of antigen-specific plasma cells is followed by somatic hypermutation, clonal selection and affinity maturation to ensure the production of the best antibodies for the job, which are then released to tackle an infection
Once the infection has been cleared, some plasma cells will remain as dormant “memory” B cells:
- only the most highly-antigen specific B cells produced by affinity maturation will be selected to become memory cells
- the presence of memory cells means that immediate plasma cell proliferation and antibody production can occur at the time of the next infection
- the number of surviving memory cells increases after each reinfection, so the more times you are exposed to a particular pathogen, the better your immune response to it becomes
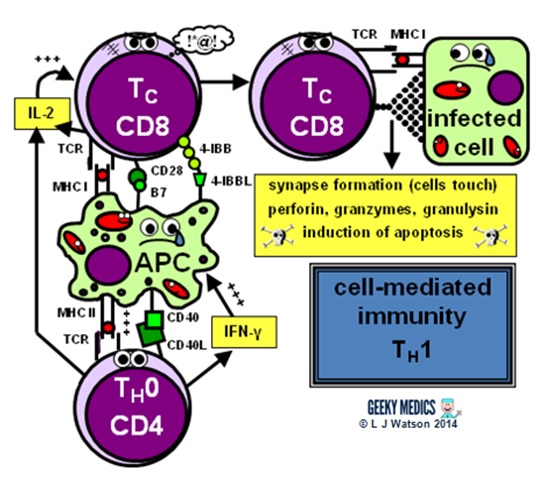
Part 4b – Cell-mediated immunity
Cell-mediated immunity is the term for a specific adaptive immune response activated by TH1 cells, which leads to activation of antigen-presenting cells and a cytotoxic T cell response.
This immune response is designed to fight intracellular infections, including viruses, some bacteria and fungi, and protozoans such as Plasmodium and Toxoplasma.
T CELL RECEPTORS
T cell receptor diversity is just as important as antibody diversity, as both systems need to be able to cover any possible infectious antigen. T cells generate this genetic diversity in essentially the same way as B cells do during their development in the bone marrow, using VDJ recombination by RAG proteins, junctional diversity and the addition of N regions, but they do not undergo class switching or somatic hypermutation during their maturation.
All immature T cells undergo a rigorous “education” in the thymus gland before they are released into the bloodstream, but this process is particularly important for cytotoxic T cells due to their destructive nature. They are “tested” with a variety of self cell antigens, and any cells which have generated a receptor that reacts to these undergo negative selection and are destroyed. This essential mechanism prevents the immune system from reacting to the body and is known as immunological tolerance or self-tolerance. In order to graduate successfully from the thymus, T cells must also express CD3 and CD4 or CD8 (but never both), and bind to self MHC complexes (but not too strongly). Only about 1% of T cells generated in the bone marrow actually make it through this process alive!
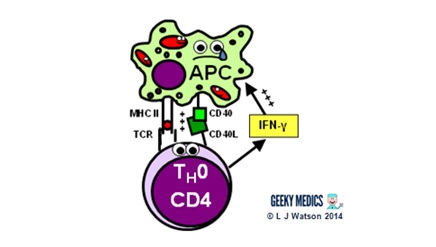
ACTIVATION OF ANTIGEN PRESENTING CELLS
The first step of the cell-mediated immune response is the activation of antigen-presenting cells:
- a TH1 cell encounters an unhappy infected antigen-presenting cell and recognises the MHC II-restricted antigen on its surface
- it then “activates” the APC by providing a CD40 ligand second signal and secreting interferon-gamma (IFNγ), a cytokine which is essential in stimulating the immune response to intracellular infections
- once activated, APCs are able to increase their production of nitric oxide and superoxide radicals, which optimises their killing mechanisms and allows them to destroy ingested pathogens much more effectively
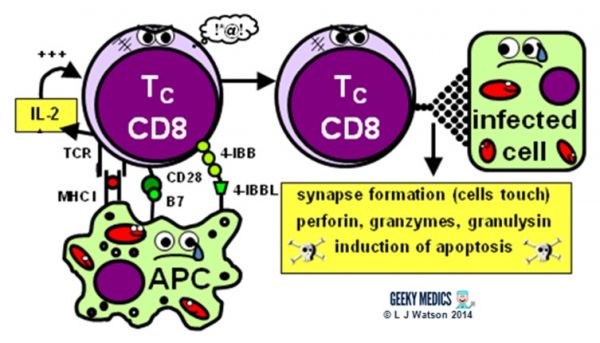
THE CYTOTOXIC T CELL RESPONSE
The next step is the activation of an antigen-specific cytotoxic T cell response:
- activated APCs present their antigen to the specific cytotoxic T cell receptor within an MHC I, along with a variety of second signals, including B7 + CD28 and/or 4-IBB + 4-IBBL
- this process is helped along by the secretion of IL-2 – a potent T cell growth factor – by TH1 cells and the cytotoxic T cells themselves
Once activated, the cytotoxic T cells are very keen to get out and start hunting and killing things. They identify infected cells by recognising the antigen displayed within MHC I on their surfaces. They then destroy these cells using one of several mechanisms:
- they classically form an immunological synapse with their target cell – this just means the cell membranes touch – and release a substance called perforin to make a hole in the cell wall. They then use this hole to release granzymes and granulysin into the cell, which induce apoptosis and DNA fragmentation.
- Fas ligand interactions between the cell surfaces can also produce apoptosis of the infected cells via the aptly named death-inducing signalling complex (DISC)
- cytotoxic T cells can also release interferon-gamma (IFNγ), which has an interesting role in viral infections, as it is able to block intracellular viral replication without killing the cell itself. This effect is very useful, as killing and lysing virally infected cells would simply let all the baby viruses out and cause the infection to spread itself even further, which is clearly suboptimal.
After the infection has been dealt with, the most antigen-specific cytotoxic T cells will remain behind as dormant memory T cells. The principles of T cell memory are essentially the same as B cell memory.
- during reinfection, only the first signal (MHC + antigen) is required to activate the cytotoxic T cell response; no second signal is necessary
- this means that any antigen-presenting cell (not just dendritic cells) can activate cytotoxic T cells directly, reducing the need for TH1 cell help and resulting in a much swifter and more efficient cell-mediated immune response.
Summary of the immune response
We’ve now covered everything in the diagram in detail – hopefully it seems a lot less scary now!
Responses to different infections
It is useful to be able to apply your knowledge of the immune response to different types of infection, especially when it crops up in exam questions. The most important differentiation to make is whether the infection is intracellular or extracellular, as this generally dictates which branch of the adaptive immune response will be activated:
- extracellular infections –> TH2 –> humoral immune response with B cells and antibodies
- intracellular infections –> TH1 –> cell-mediated immune response with activated APCs and cytotoxic T cells
Some types of pathogens can only exist as either extracellular or intracellular organisms, whilst other types can vary depending on the individual species. There are also unique variations in aspects of the immune response for some organisms.
BACTERIA
- bacterial infections trigger the classic immune response as described in the main article above
- bacterial infections are usually extracellular
- however, some bacteria do choose to exist as intracellular organisms; examples of these include Neisseria, Salmonella, Chlamydia and Mycobacteria
VIRUSES
- viral infections are intracellular and therefore handled by cell-mediated immunity
- interferons are a family of cytokines which act as the equivalent of complement in viral immunity, and also have additional unique functions. For example, cytotoxic T cells release interferon-gamma, which inhibits viral replication within infected cells without damaging the cells themselves.
- new baby viruses are released from cells as part of the spread of a viral infection, and viral antigens are also expressed on the surfaces of infected cells. This means that some aspects of humoral immunity are also useful in viral infections. Antibodies are able to bind to viral antigens in order to neutralise and opsonise the baby viruses after they are released, limiting the spread of infection.
- natural killer cells also play a vital role in viral immunity, as they recognise and destroy infected cells which have either expressed antigen on their surfaces or lost their inhibitory MHC I
FUNGI
- the normal immune response is able to deal with fungal infections very swiftly and effectively
- fungal infections are usually extracellular and therefore dealt with by humoral immunity
- less commonly, fungi opt to “break the mould” (sorry) and become intracellular – examples of these include Histoplasma, Cryptococcus and Pneumocystis, all of which are well known to cause opportunistic infections in immunosuppressed patients lacking the cell-mediated immunity vital in tackling such organisms
- macrophages and other phagocytes are very important in fungal immunity
PROTOZOANS
- our immune response to protozoa isn’t that great. This is probably because many species have evolved hundreds of clever protective mechanisms which turn the immune system to their advantage. Examples of protective mechanisms include resistance to phagocytosis and complement lysis, antigen variation, antigen shedding and even direct modifications of host immune mechanisms.
- examples of extracellular protozoa include Giardia, which infects the intestines and may have caused many of you the joys of traveller’s diarrhoea; Entamoeba, which causes dysentery and nasty amoebic liver abscesses; and Trypanosoma, which causes sleeping sickness and featured in an excellent episode of House.
- other protozoa are intracellular organisms. Examples include Plasmodium, which occupies red blood cells and liver cells to cause malaria; Leishmania, which survives inside phagocytes after being ingested and affected many soldiers during the conflicts in Iraq and Afghanistan; and Toxoplasma, which lives in many body tissues and can cause “crazy cat lady syndrome” amongst other things.
WORMS (a.k.a HELMINTHS)
- worms are very big compared to other infective organisms and are obviously always extracellular – common examples include Schistosoma, which spreads worms through the bloodstream to vital organs; Onchocercha which causes river blindness; and Taenia tapeworms which can cause malnutrition and cysticercosis
- TH2 cells and humoral immunity form the basis of the body’s immune response to parasitic worms
- eosinophils and IgE are also very important in killing helminths, as alongside promoting a powerful inflammatory response, they appear to bind to the opsonising antibodies on the worm’s skin in order to subsequently dissolve it and kill it
The end
I hope you found this guide helpful and fun. I certainly enjoyed creating the little immune cells and telling their story (perhaps a bit too much actually). It was a mammoth undertaking to write this thing, and while I have tried to be as thorough and accurate as possible if any of you clever folks out there have noticed any mistakes/miscommunications/other cock-ups please do let me know so I can correct them for the benefit of everyone else. In return, you shall receive the reward of your name immortalised below and a Geeky Medics gold star…
- many thanks to Colin Hill for helpfully highlighting a mix-up in Part 3!
References
- Immune System Anatomy. By OpenStax College [CC BY 3.0], via Wikimedia Commons. Available from: [LINK]
- Andy Gennery’s totally excellent immunology lectures given at Newcastle Medical School in 2009 and 2011 (I hope I did them justice)
- Murphy K; “Janeway’s Immunobiology, 8th Edition”, Garland Science.
- ParaSite – Parasites and Pestilence by Stanford University. Available from: [LINK]
- Patient UK. Full Blood Count. Available from: [LINK]