- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Oxygen (O2) is an essential molecule in the human body. It is the final electron acceptor in the electron transport chain, located in the mitochondria, and so has a key role in the production of aerobic energy – i.e. adenosine triphosphate (ATP). A constant supply is therefore required to tissues around the body, and this is achieved by the carriage of oxygen in the bloodstream.
Oxygen enters the bloodstream via the lungs, where it diffuses across the alveolar epithelium and pulmonary capillary endothelium to reach the pulmonary circulation.
Once the oxygen has entered the pulmonary circulation, it is carried in the blood to target tissues in two distinct forms:
- Bound to haemoglobin (around 98% of total blood oxygen content)
- Directly dissolved in the plasma (only around 2% of total blood oxygen content) 1
Two measurements commonly used in clinical practice to investigate how oxygen is being carried in the blood are the partial pressure of oxygen (PO2) and the oxygen saturation (SO2). The partial pressure of oxygen reflects the amount of oxygen dissolved directly in the plasma, while the oxygen saturation reflects the proportion of haemoglobin that has oxygen bound to it.
Haemoglobin
Haemoglobin is a tetramer, meaning it is made up of four subunits. Each subunit is formed of a globin polypeptide chain and an associated haem group (a porphyrin ring with a central iron atom). Each iron atom, and therefore each subunit, can reversibly associate with a single oxygen molecule.
There are a variety of structurally distinct subunits that combine to form different types of haemoglobin. Normal adult haemoglobin (haemoglobin A) makes up about 97% of adult haemoglobin and is comprised of 2 α and 2 β subunits. Fetal haemoglobin has a different subunit make-up and the reasons for this are discussed later.
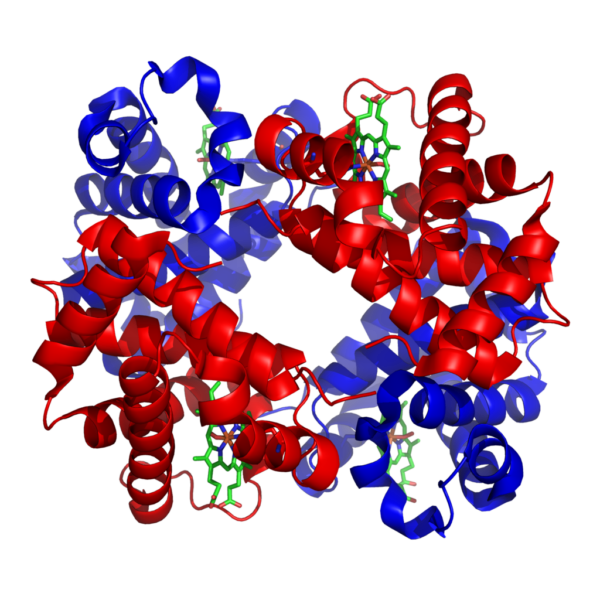
Haemoglobin-oxygen binding
Each haemoglobin subunit can bind a single oxygen molecule, so each haemoglobin molecule can associate with between 0 and 4 oxygen molecules at any one time.
When an oxygen molecule binds to a haem group, a conformational change occurs in the related globin chain structure. As the globin chains are closely linked, a change in the shape of one subunit is also transmitted to the other subunits. Oxygen binding to one haemoglobin subunit acts to increase the remaining subunits’ affinity for oxygen. Deoxygenated haemoglobin exists in a ‘tense’ (T) conformation, with a low affinity for oxygen. As oxygen begins to bind to haem groups, the haemoglobin moves into a ‘relaxed’ (R) state, allowing further oxygen molecules to bind more easily. This process is referred to as co-operativity.2
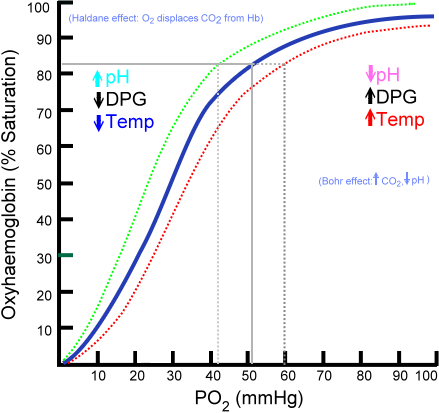
This co-operativity between the subunits results in the characteristic sigmoidal oxygen-haemoglobin dissociation curve shown in Figure 2.
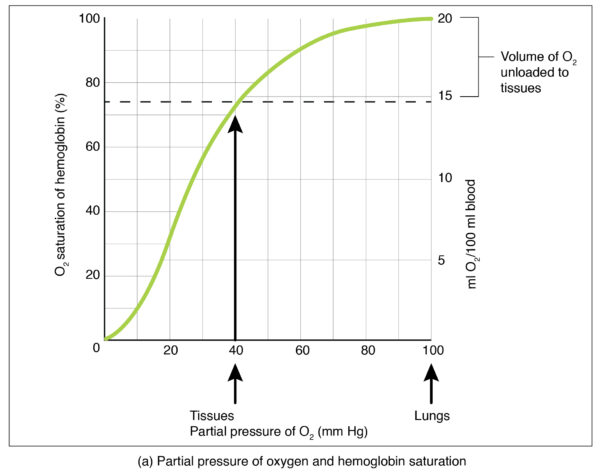
Haemoglobin-oxygen affinity
As shown by the oxygen-haemoglobin dissociation curve above, the amount of oxygen bound to haemoglobin (the oxygen saturation) is affected by the partial pressure of oxygen (PaO2) in the blood. However, this relationship and the shape of the curve are not constant, as the affinity of haemoglobin for oxygen is affected by the physiological environment.
Some important factors which affect haemoglobin-oxygen affinity are discussed below.
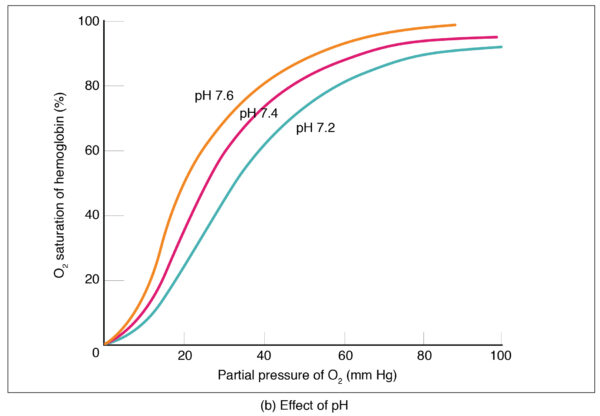
pH
Low pH, a feature of tissues with high metabolic activity, reduces the affinity of haemoglobin for oxygen, shifting the curve to the right. The reduced affinity means that more oxygen is offloaded in metabolically active tissues, where the oxygen requirement is highest. The reduction of haemoglobin-oxygen affinity at low pH is known as the Bohr effect.
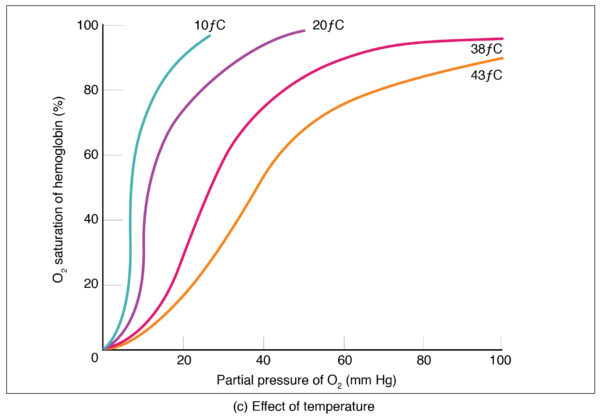
Temperature
Increased temperature reduces haemoglobin’s affinity for oxygen, shifting the curve to the right. This has an important effect during exercise when active muscle rises in temperature and therefore triggers increased oxygen offloading.
2,3-DPG
2,3- diphosphoglycerate is an intermediate product of glycolysis, found in red blood cells. 2,3-DPG reduces the affinity of haemoglobin for oxygen, shifting the curve to the right. Increased 2,3-DPG is found in anaemia and at altitude, which is important in the prevention of tissue hypoxia.2
Fetal haemoglobin
Fetal haemoglobin needs to behave differently to adult haemoglobin in order to facilitate oxygen delivery from mother to fetus across the placenta. This is achieved by structural differences between adult and fetal haemoglobin. Fetal haemoglobin is a tetramer like the adult form, but the 2 β subunits are replaced by 2 γ subunits, creating an α2γ2 structure. The result of this subunit replacement is that fetal haemoglobin has a higher affinity for oxygen than adult haemoglobin does. This facilitates delivery of oxygen across the placenta from the maternal circulation (lower affinity adult haemoglobin) to the fetal circulation (higher affinity fetal haemoglobin).
Clinical relevance: Carbon monoxide poisoning
Carbon monoxide is a colourless, odourless gas which can be released from faulty boilers or exhaust fumes as well as being present in polluted air and cigarette smoke. Its pathological effects result from its ability to impair haemoglobin oxygen carriage. Carbon monoxide binds to the haem groups of haemoglobin to form carboxyhaemoglobin, but with a roughly 210 times higher affinity than oxygen does. The increased affinity that carbon monoxide has for haemoglobin means that even low concentrations can displace oxygen from its binding sites and markedly reduce oxygen delivery to tissues. Carbon monoxide binding also shifts the oxygen haemoglobin dissociation curve to the left, reducing the ability of any bound oxygen to dissociate in the tissues and leading to tissue hypoxia.
The low oxygen saturation resulting from carbon monoxide poisoning is often not detected by pulse oximetry, as most pulse oximeters cannot differentiate between oxyhaemoglobin and carboxyhaemoglobin. Oxygen saturation measurements via an arterial blood gas, however, should reveal the true oxygen saturation. PaO2 will remain unaffected by carbon monoxide toxicity.
Carbon monoxide poisoning can cause a range of symptoms, depending on concentration and length of exposure (and therefore the carboxyhaemoglobin concentration). Mild symptoms include headaches, nausea and vomiting and lethargy but high concentrations or acute toxicity can result in seizures, coma or even death. 3
References
- Pittman RN. Regulation of Tissue Oxygenation: Chapter 4, Oxygen Transport. 2011. Available from: [LINK]
- Thomas C, Lumb AB. Physiology of haemoglobin. 2012. Available from: [LINK]
- Blumenthal I. Carbon monoxide poisoning. 2001. Available from: [LINK]
- Zephyris at the English language Wikipedia, CC BY-SA 3.0. Available from: [LINK]
- OpenTextBC. The respiratory system: transport of gases. Licence: CC 4.0. Available from: [LINK]




