- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Blood pressure (BP) can provide insight into the workings of the heart and vessels of the body. BP is controlled by a variety of complex physiological mechanisms which allow both short-term adaptation and longer-term maintenance of BP within a normal range.
Blood pressure which is too high or too low can lead to a wide range of pathology (e.g. ruptured blood vessels, reduced perfusion to organs) and therefore the mechanisms which maintain BP homeostasis need to be robust.
Blood pressure can be measured in a number of different ways, with the most common being systolic and diastolic blood pressure:
- Systolic blood pressure (SBP) represents the pressure in the blood vessels when the heart contracts (systole).
- Diastolic blood pressure (DBP) represents the pressure in the blood vessels between heartbeats (diastole).
Mean arterial blood pressure (MABP) is another method of assessing blood pressure:1
- Mean arterial blood pressure = Cardiac output x Systemic vascular resistance
MABP can be calculated from the SBP and DBP using the following formula:
- MABP = DBP + (Pulse pressure / 3)
- Pulse pressure is calculated by subtracting DBP from SBP (i.e. SBP – DBP)
Rapid control of blood pressure
Baroreceptor reflex
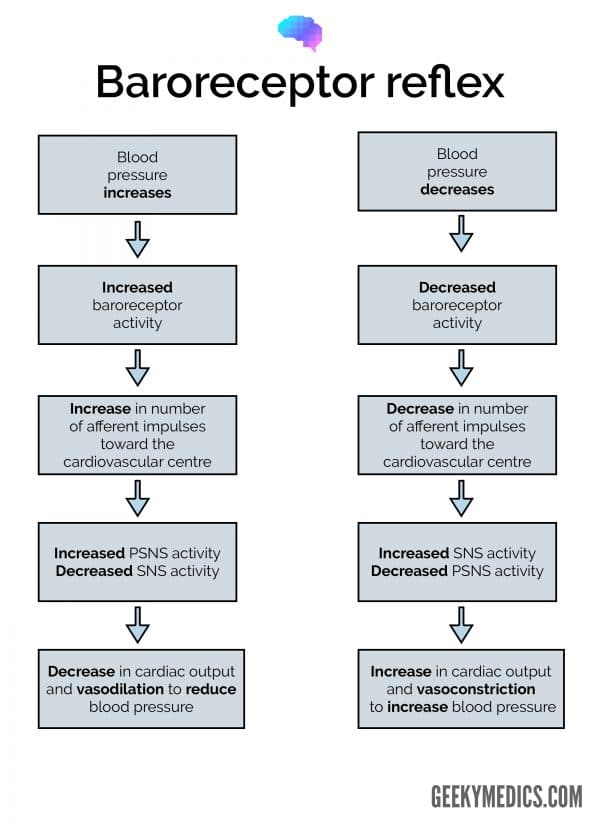
The baroreceptor reflex is a neurally-mediated reflex that regulates blood pressure in the short term. This reflex is crucial for maintaining blood pressure throughout the day, and in its absence, even a slight change in posture could lead to significant changes in blood pressure.
There are mechanoreceptors known as baroreceptors located in the aortic arch and carotid sinus, which constantly monitor the MABP and pulse pressure.1 Increases in arterial pressure result in increased baroreceptor activity, increasing the firing rate in the associated afferent neurons, carrying this information to the cardiovascular centre in the medulla.1,2
In response to this, the parasympathetic nervous system (PSNS) activity increases and the sympathetic nervous system (SNS) activity decreases. The net effect is reduced heart rate and systemic vasodilatation, collectively reducing MABP.
Conversely, when blood pressure decreases, there is less baroreceptor activation meaning the impulse rate of the afferent fibres decreases, causing the cardiovascular centre to increase SNS outflow and decrease PSNS outflow.2 The net effect of this is increased cardiac contractility, increased heart rate and increased systemic vasoconstriction, causing an overall increase in blood pressure.1,2
Intermediate and long-term regulation of BP
Renin-angiotensin-aldosterone system (RAAS)
The renin-angiotensin-aldosterone system (RAAS) is an essential component of blood pressure regulation that acts to increase blood volume and increase systemic vascular resistance.1 This system is dependent on hormonal changes which induce transcription of genes to produce vasoactive proteins, making it a slower means of controlling blood pressure than the baroreceptor reflex.3
The RAAS system starts with renin, a hormone released from granular cells in the juxtaglomerular apparatus, a specialised structure involving parts of the distal convoluted tubule (DCT) and the adjacent afferent arteriole of the glomerulus.4
Renin is released in response to increased concentration of salt in the blood, reduction in renal blood flow, or stimulation from the sympathetic nervous system acting on beta-1 receptors.1
Renin converts angiotensinogen, a protein synthesised by the liver, into angiotensinogen I, which is subsequently converted by angiotensinogen-converting enzyme (ACE) into angiotensin II. Angiotensin II causes vasoconstriction in the systemic circulation and the renal microvasculature, preferentially constricting the efferent arteriole.5
ACE, which is found primarily in the lungs, also rids the body of a vasodilator called bradykinin, causing further vasoconstriction.1,6
Importantly, angiotensin II increases salt reabsorption at the level of the kidney and does so indirectly through the activation of aldosterone released from the zona glomerulosa of the adrenal cortex.1,7,8 Increased salt retention subsequently increases plasma volume and blood pressure.
Angiotensin II is also capable of increasing plasma volume through the stimulation of thirst and antidiuretic hormone (ADH), another regulator of blood pressure, which will be discussed shortly.1,6
Aldosterone acts on the principal cells found in the DCT and collecting duct of the nephron, increasing Na+ reabsorption while simultaneously increasing K+ secretion into the tubules.3,7
Aldosterone-mediated salt resorption is also linked to H+ secretion.1 Given the ability of aldosterone to increase the volume of the extra-cellular fluid compartment and thus BP, several common anti-hypertensive medications aim to decrease blood pressure through inhibition of aldosterone formation.
Antidiuretic hormone (ADH)
Antidiuretic hormone, also known as vasopressin, is involved in the control of blood pressure. ADH is made by cell bodies located in the hypothalamus and released from the adjacent posterior pituitary.1,6 The following physiological changes trigger ADH release:
- an increase in plasma osmolarity (detected by osmoreceptors in the hypothalamus)
- a reduction in blood volume
- an increase in the levels of angiotensin II
ADH increases water reabsorption by binding to V2 receptors, subsequently anchoring water channels known as aquaporins to the apical membrane of its target, principal cells in the collecting duct and DCT of the kidney.1,2 These aquaporins, named AQP-2 channels, are accountable for the variable H2O permeability at the distal part of the nephron, as water cannot pass through without them.1
When someone becomes dehydrated, the osmolarity of the extracellular fluid increases, leading to ADH release from the posterior pituitary.6 Water is then reabsorbed at an increased rate at the level of the kidney, ultimately acting to increase the intravascular fluid volume. This increases blood pressure through an increase in venous pressure, thereby boosting venous return to the heart, and increasing cardiac output.
ADH also acts as a vasoconstrictor targeting V1 receptors on vascular smooth muscle at high concentrations, such as in response to haemorrhagic shock.6
Other regulators of blood pressure
Low-pressure baroreceptors
Low-pressure baroreceptors, in contrast to the high-pressure baroreceptors discussed previously, are found in the venous system, atria and pulmonary arteries.3 They respond to changes in plasma volume, modulating blood pressure via various mechanisms.
Atrial natriuretic peptide
Atrial natriuretic peptide (ANP) is a vasoactive peptide released from the atria in response to a rise in atrial pressures, which in turn are linked to venous pressure.6
ANP lowers blood pressure, primarily by vasodilation and the inhibition of sodium reabsorption by the kidney, the latter having a diuretic effect.1.3 This system increases sodium excretion in part through the opposition of the renin-angiotensin-aldosterone system, inhibiting renin and aldosterone release.1 ANP has also been shown to have inhibitory effects on vasopressin.1
Table 1. A summary of vasoactive compounds.
| Vasoactive compound | Site of production | Effects on vasculature | Effects on extracellular fluid (ECF) levels |
|
Angiotensin II |
Various sites: ACE gives rise to angiotensin II (mostly in the lungs) |
Vasoconstriction |
Increases ECF volume Increases sodium reabsorption independently, and stimulates aldosterone and ADH production |
|
Aldosterone |
Adrenal glands (cortex) |
– |
Increases ECF volume by increasing sodium reabsorption |
|
Anti-diuretic hormone (ADH) |
Hypothalamus (released from the posterior pituitary) |
Vasoconstriction |
Increases ECF volume by increasing H2O reabsorption |
|
Anti-natriuretic peptide (ANP) |
Cardiomyocytes |
Vasodilatation |
Decreases ECF volume by reducing sodium reabsorption |
How do vasoactive compounds change SVR and BP?
Vasoactive compounds often modify the amount of resistance in the systemic circulation (systemic vascular resistance – SVR) by targeting arterioles, the smallest arterial vessels.
The smooth muscle in these vessels contains several receptors, which when bound to, give rise to either of the following responses, depending on receptor type:
- Stimulation of smooth muscle contraction: decreasing vessel diameter and increasing systemic vascular resistance
- Inhibition of smooth muscle: increasing vessel diameter and reducing systemic vascular resistance
Changes to the diameter of these small vessels occur throughout the body, increasing the arteriolar tone. When the area through which blood passes decreases, blood pressure increases.
To consolidate this concept, let us examine the effect of angiotensin II on the arterioles. Angiotensin II binds to AT1 receptors on arterioles, triggering an array of intracellular processes that lead to smooth muscle contraction in targeted vessels.1 This reduces the area through which blood can flow, increasing systemic vascular resistance (SVR), and, therefore blood pressure (BP).
Key points
- Blood pressure regulation is a complex process, regulated by several mechanisms that work in unison to maintain homeostasis.
- Rapid adjustments in blood pressure are typically neurally mediated by the baroreceptor reflex.
- Intermediate and long term regulation of blood pressure is predominantly mediated by vasoactive compounds.
References
- Sherwood L. Human Physiology: From Cells to Systems – 9th Edition. Published in 2016. Available from Cengage Learning.
- Mulroney S, Myers A, Netter FH, Machado CA, Craig JA, Perkins JA. Netter’s Essential Physiology. Published in 2009. Available from Elsevier Inc.
- Costanzo LS. Physiology- 6th Edition. Published in 2018. Available from Elsevier.
- Unknown author. Histology @ Yale: Juxtaglomerular apparatus. Available from: [LINK]
- Joannidis M, Hoste E. Angiotensin inhibition in patients with acute kidney injury: Dr. Jekyll or Mr. Hyde. 2018. Intensive care med. https://doi.org/10.1007/s00134-018-5223-8
- Boron WF, Boulpaep EL. Medical Physiology. Published in 2012. Available from Elsevier Inc.
- Scott JH, Menouar MA, Dunn RJ. Physiology, Aldosterone. StatPearls Publishing. Published in 2020. Available from: [LINK]
- Hall JE. Guyton and Hall Textbook of Medical Physiology – 13th Edition. Published in 2016. Available from Elsevier Inc.




