- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
The autonomic nervous system (ANS) is a branch of the peripheral nervous system (PNS) that regulates the function of the viscera. It innervates smooth muscle as well as glands and is further divided into the parasympathetic and sympathetic systems.
The ANS has an essential role in controlling internal organ function, regulating heart rate, blood pressure, micturition, sweating, sexual function and gastrointestinal transit (digestion and defecation).
Broadly speaking the two branches of the ANS can be thought of as opposing or antagonistic systems, with the sympathetic arm acting as the exciter (eliciting the “fight or flight” response) and the parasympathetic as the suppressor (eliciting the “rest and digest” response).
Overview of the basic organisation of the nervous system
- Central nervous system (CNS): brain and spinal cord
- Peripheral nervous system (PNS): peripheral nerves, ganglia and sensory receptors
- Somatic nervous system (SNS): conscious motor control (e.g. purposeful movement of skeletal muscle)
- Autonomic nervous system (ANS): involuntary motor control of smooth muscle and glands
- Sympathetic ANS: “fight or flight” response
- Parasympathetic ANS: “rest and digest” response
- Enteric nervous system (ENS/intramural plexus): semiautonomous nervous system that controls digestion with input from the ANS
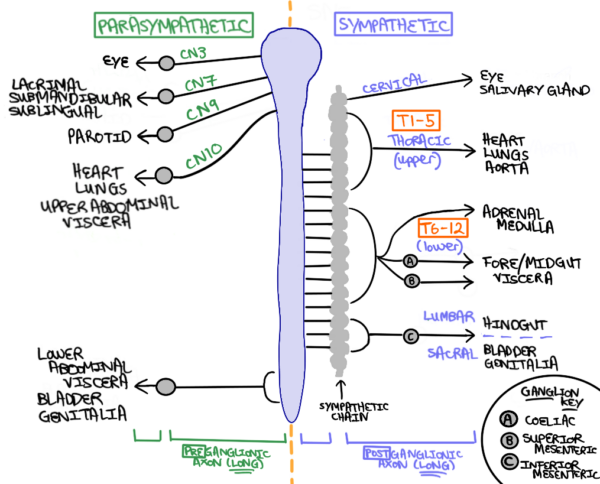
Sympathetic autonomic nervous system
The primary function of the sympathetic ANS is the “fight or flight” response, designed to prepare the body for stressful situations.
The combined sympathetic response optimises blood flow to the cardiovascular, respiratory and musculoskeletal systems to maximise the delivery of oxygen to tissues.
Blood flow is diverted away from the gut to increase oxygen delivery to skeletal muscle, and sweating is used to dissipate the heat generated during exertion to maintain core body temperature.
Key actions of sympathetic activation
- Increased cardiac output (via increased heart rate, stroke volume and myocardial contractility)
- Arterial vasoconstriction (increased mean arterial pressure)
- Bronchodilation
- Pupillary dilation
- Inhibition of salivation and peristalsis
- Mobilisation of glucose stores (glycogenolysis and gluconeogenesis)
- Relaxation of the bladder and contraction of the internal urinary sphincter (urinary retention)
- Penile ejaculation
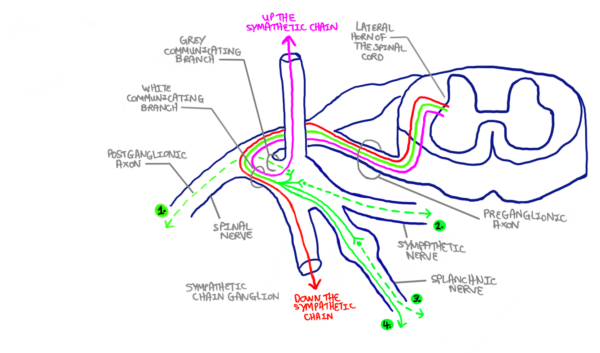
Anatomy
Sympathetic pre-ganglionic fibres arise from neurones in the lateral horn of the spinal cord between T1-L2/3 (the thoracolumbar ANS outflow).
Pre-ganglionic sympathetic fibres are short and exit the spinal cord via the ventral root before joining the spinal nerve. Here they depart via the white communicating branch (myelinated) before taking one of four described routes (Figure 2):
- Pre-ganglionic axons can synapse with a para-vertebral ganglion in the sympathetic chain: This can happen either at the level of entry or in a superior or inferior sympathetic chain ganglion (hence the term “chain”). The post-ganglionic axon then departs via the grey communicating branch (unmyelinated) and re-joins the spinal nerve, travelling within the PNS to the end-organ (i.e. skin, blood vessels, sweat glands).
- Pre-ganglionic axons can synapse within the para-vertebral ganglion in the sympathetic chain but instead, exit via a sympathetic nerve to innervate the organs of the thoracic cavity (e.g. heart and lungs).
- Pre-ganglionic axons can pass through the sympathetic chain without synapsing and exit via the splanchnic nerve. This synapses in a pre-vertebral ganglion (e.g. coeliac, superior mesenteric, inferior mesenteric) and innervates the organs of the abdominopelvic cavity.
- Pre-ganglionic axons can travel via the splanchnic nerve directly to the adrenal medulla without synapsing.
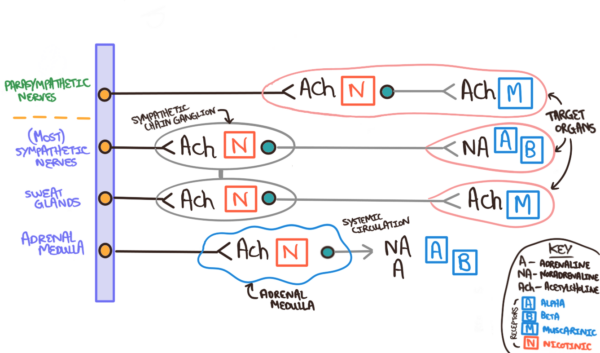
Bar some notable exceptions, acetylcholine (ACh) is the neurotransmitter at the pre-ganglionic synapse (acting on nicotinic receptors) and noradrenaline (NA) is the neurotransmitter at the post-ganglionic synapse (acting on adrenergic receptors) to stimulate an end-organ response.
The first exception to this rule is the adrenal medulla, which is directly innervated by the pre-ganglionic axon. At this synapse ACh is the neurotransmitter acting on nicotinic receptors, resulting in the secretion of adrenaline and noradrenaline into the blood.
Sweat glands are another exception to this rule as ACh is the neurotransmitter at both the pre-synaptic (acting on nicotinic receptors) and post-ganglionic synapses (acting on muscarinic receptors). The differences between these axons can be seen in Figure 3.
Parasympathetic autonomic nervous system
The primary function of the parasympathetic ANS is to regulate the body’s “rest and digest” system. It also forms a vital role in antagonising the sympathetic system to restore normal physiology after sympathetic arousal.
Key actions of parasympathetic activation
- Reduces heart rate
- Bronchoconstriction and increased mucous production
- Pupillary constriction
- Stimulating salivation and peristalsis
- Fuel storage (increased insulin secretion)
- Contraction of the bladder and relaxation of the internal urinary sphincter (urination)
- Penile erection
Anatomy
Parasympathetic pre-ganglionic neurones exit the spinal cord via the cranial and sacral spinal nerves (craniosacral parasympathetic outflow).
The cranial parasympathetic outflow (CN III, VII, IX & X) innervates the viscera of the upper body (until just before the transverse colon’s splenic flexure), and sacral spinal nerves innervate the lower half of the body via the pelvic splanchnic nerves.
Pre-ganglionic fibres are long and synapse close to their effector organ resulting in a short post-ganglionic neurone. ACh is the neurotransmitter at both the pre-ganglionic and post-ganglionic neurones, acting on nicotinic and muscarinic receptors respectively (as seen in Figure 3).
Muscarinic and adrenergic receptors
Nicotinic and muscarinic receptors are the two different types of ACh receptors.
Nicotinic receptors are ionotropic and are agonised by nicotine (as suggested) and ACh. They are the receptor in the pre-ganglionic synapse of the autonomic ganglia, in addition to being found at the neuromuscular junction and in the brain.
Muscarinic receptors are G-protein coupled receptors that are agonised by muscarine (a fungal alkaloid) and ACh. They are the receptor at the post-ganglionic synapse of parasympathetic nerves and sweat glands (sympathetic). Five different sub-types of muscarinic receptors exist which are found within different tissues:
- M1 receptors: secretory glands and CNS
- M2 receptors: heart
- M3 receptors: smooth muscles of bronchioles and arterioles
- M4/5 receptors: CNS
Adrenergic receptors are also G-protein coupled receptors that are agonised by catecholamines (i.e. adrenaline, noradrenaline). There are five sub-types of adrenergic receptors (A1/2 and B1/2/3) which act as the receptor at the post-ganglionic synapse of sympathetic nerves (except for sweat glands).
As a rule of thumb, catecholamine activation of even-numbered adrenoreceptor subtypes (i.e. A2/B2) usually results in an inhibitory end organ response, whilst odd-numbered subtypes (A1, B1/3) are excitatory. The function of all these receptors is summarised below in Table 1 in addition to their clinical/pharmacological relevance.
Table 1. Location and function of adrenergic and cholinergic receptors
|
|
Sympathetic stimulation | Parasympathetic stimulation | Clinical relevance |
|
Eyes (iris) |
A1 receptors Dilator pupillae (outer radius muscle) contracts resulting in mydriasis (pupillary dilation)
|
Muscarinic (M3) receptors Sphincter pupillae (inner circular muscle) contracts resulting in miosis (pupillary contraction) |
Topical mydriatic agents for pupillary dilation during retinal assessment
Impaired near vision is a notable side effect of topical atropine administration due to impaired ciliary muscle contraction Glaucoma treatment:
|
|
Eye (ciliary muscle) |
B2 receptors Ciliary muscles relax facilitating distance vision |
Muscarinic (M3) receptors Ciliary muscles contract facilitating near vision Increased outflow of aqueous humour into the canal of Schlemm (decreasing IOP) |
|
|
Eye (ciliary body epithelium) |
B1 receptors Increased production of aqueous humour (increasing IOP) |
– |
|
|
Heart |
B1 receptors (SA node and myocardium) Increased HR and myocardial contractility |
Muscarinic (M2) receptors (via vagus nerve) Decreased HR
|
Bisoprolol (selective B1 antagonist) is used as an antihypertensive agent and to reduce myocardial oxygen demand in ischemic heart disease (by decreasing heart rate and myocardial contractility) IV atropine (muscarinic antagonist) used in the emergency treatment of symptomatic bradyarrhythmias |
|
Arterioles (skin and abdominal viscera) |
A1 receptors (high level of tissue expression) Strong vasoconstriction |
– |
Metaraminol (adrenergic agonist with A > B action). Increases the force of myocardial contractions and acts as a potent peripheral vasoconstrictor to increase mean arterial pressure. Commonly used for the treatment of acute hypotension due to loss of vasoconstrictor tone during spinal anaesthesia Doxazosin (A1 antagonist) used to treat hypertension |
|
Arterioles (skeletal muscle) |
A1 receptors (low level of tissue expression) Weak vasoconstriction B2 receptors Vasodilation |
– |
|
|
Lungs |
B2 receptors (smooth muscle lining the bronchial tree) Bronchodilation |
Muscarinic (M3) receptors (smooth muscle lining the bronchial tree) Bronchoconstriction |
Ipratropium (M antagonist) and salbutamol (B2 agonists) are used in the treatment of obstructive respiratory disease to induce bronchodilation. |
|
GI tract |
A1/A2/B2 receptors (GI tract wall) Decreased tone A1 receptors (GI tract sphincters) Contraction |
Muscarinic (M3) receptors Increase GI tract wall tone, sphincter relaxation and increased GI secretions |
The enteric nervous systems activity can be modified by the ANS as described |
|
Salivary gland |
A1 receptors Constriction of vessels resulting in thick saliva |
Muscarinic (M1) receptors Salivation |
– |
|
Pancreas |
A2 receptors Decreased insulin and exocrine digestive enzyme secretion |
Muscarinic receptors Increased insulin and digestive enzyme secretion |
– |
|
Liver |
A1/B2 receptors Increased glycogenolysis (breakdown of glycogen into glucose) and gluconeogenesis (creation of glucose) |
– |
– |
|
Adipose tissue |
B3 receptors Lipolysis, thermogenesis in skeletal muscle |
– |
– |
|
Skin (piloerector muscles) |
A1 receptors Piloerection (hairs stand on end / “goosebumps”) |
– |
– |
|
Sweat glands (axilla, palms, soles, genitalia) |
A1 receptors Sweating |
– |
Terazosin (A1 antagonist) used to treat hyperhidrosis |
|
Kidney |
B1 receptors Increased renin release |
– |
– |
|
Urinary system |
B2 receptors (detrusor muscle) Relaxation (urinary retention) A1 receptors (internal urinary sphincter Contraction (stops urinary flow) |
Muscarinic (M3) receptors Contraction of the detrusor muscles and relaxation of the urinary sphincter (urination) |
Tamsulosin (A1 antagonist) is used to treat poor urinary flow in benign prostatic hyperplasia |
|
Uterus (during pregnancy) |
A1 receptors Contraction B2 receptors Relaxation |
Muscarinic receptors Contraction |
Salbutamol/terbutaline (B2 agonists) can be used as a tocolytic agent in premature labour, reducing uterine contractions |
|
Adrenal medulla |
Nicotinic receptors Increased secretion of adrenaline and noradrenaline |
– |
– |
Clinical relevance: Valsalva manoeuvre
A Valsalva manoeuvre can be performed by forcing expiration against a closed glottis for ~15 seconds. In clinical settings, this is often achieved by asking patients to try and inflate an empty syringe.
The resulting increase in intrathoracic pressure has a four-phase effect on cardiovascular haemodynamics involving parasympathetic vagal nerve stimulation:
- Increased intrathoracic pressure squeezes the pulmonary vessels increasing venous return to the left heart. This causes a transient increase in stroke volume (SV) (via the Frank-Starling mechanism) increasing cardiac output (CO) and mean arterial pressure (MAP). Baroreceptors in the aortic arch detect this increase in MAP and produce transient bradycardia via the baroreceptor reflex.
- Increased intrathoracic pressure prevents venous return to the right heart due to squeezing of the vena cava. This reduces cardiac preload, in turn reducing SV, CO and MAP. The baroreceptor reflect detects this reduced MAP causing an increase in heart rate.
- Intrathoracic pressure reduces and normalises as the Valsalva manoeuvre is ceased at ~15 seconds. This causes an increase in pulmonary venous sequestration due to increased intrathoracic venous capacitance, further decreasing SV, CO and MAP. This results in a further increase in heart rate through the baroreceptor reflex.
- As left ventricular preload is restored there is an increase in SV, CO and MAP. As the heart rate is still elevated there is an overshoot in MAP. This is rapidly corrected by the baroreceptor reflex causing a reflex bradycardia (via parasympathetic vagal nerve stimulation). This mechanism returns normal cardiovascular physiology
The Valsalva manoeuvre is an effective non-pharmacological first-line treatment for supraventricular tachycardia. The intense parasympathetic vagal stimulation in phase four slows conduction through the AV node and can often terminate supraventricular tachycardias. If this fails, pharmacological methods (i.e. IV adenosine) are required.
Patients with autonomic dysfunction (e.g. diabetic autonomic neuropathy, heart transplant recipients and patients with high spinal cord injuries) lack the normal baroreceptor reflex. As a result, their heart rate will remain constant throughout the Valsalva manoeuvre and their MAP will continue to fall until intrathoracic pressure is released. In this context, Valsalva manoeuvres can be used as a diagnostic tool for autonomic neuropathy.
Reviewer
Dr Maximilian Ralston
ST5 Intensive Care Medicine
Editor
Dr Chris Jefferies
References
- Betts JG, Desaix P, Johnson E, Johnson JE, Korol O, Kruse D, et al. Chapter 15: The Autonomic Nervous System. In: Anatomy & Physiology. OpenStax; 2013. p. 655–77.
- Brigham Young University. Autonomic Nervous System. BIO264: Anatomy & Physiology. 2022. Available from: [LINK]
- Chambers D, Huang C, Matthews G. Chapter 59: Autonomic Nervous System. In: Basic Physiology for Anaesthetists. 2nd edition. Cambridge: Cambridge University Press; 2019. p. 267–71.
- Johnson BK. Chapter 19: Physiology of the Autonomic Nervous System. In: Basic Sciences in Anesthesia. Springer; 2018. p. 355–64.
- Khanna S. Chapter 17: Cardiovascular Physiology. In: Basic Sciences in Anesthesia. Springer; 2018. p. 299–327.




