- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Diabetes can manifest itself through several ophthalmic conditions, grouped under the term diabetic eye disease.1
Diabetic retinopathy (DR) is the most prevalent of these and is characterised by damage to the microvasculature supplying the eye due to chronically high glucose levels. The resulting insult to retinal cells can lead to a progressive deterioration in vision through various mechanisms and can lead to blindness.
Cataracts and cranial nerve palsies are examples of other ophthalmic complications associated with diabetes. Diabetic individuals are also at an increased risk of retinal artery/vein occlusions.
Epidemiology
DR is the most common cause of blindness in the working-age demographic of the UK.
There are approximately 3.5 million people diagnosed with diabetes in the UK.2
Almost all patients with type 1 diabetes will have some degree of retinopathy within 20 years of diagnosis.3
Many patients with type 2 diabetes may already have some signs of retinopathy at diagnosis, after going through an asymptomatic period of hyperglycaemia.
Aetiology
Diabetes is characterised by high blood glucose levels because of the body’s inability to produce insulin and/or resistance of the body to insulin.
Chronic hyperglycaemia causes blood vessels, including those supplying the retina, to weaken and rupture; the vessel walls may dilate resulting in microaneurysms or small haemorrhages.
The damaged pericytes and erythrocytes increase vascular permeability. Lipoproteins, lipids and other products carried by blood are therefore able to leak out and cluster onto the retina as hard exudates.
As blood flow becomes increasingly compromised, regions of the retina are starved of oxygen. This hypoxia is thought to stimulate the release of mediators such as vascular endothelial growth factor (VEGF) which promotes neovascularization. However, these new vessels are poorly formed and easily rupture resulting in bleeding.
Neovascularization into the vitreous humour may culminate in widespread vitreous haemorrhage causing sudden and complete visual loss. Fibrovascular bundles can lead to fibrosis and, in turn, retinal traction. This can result in retinal detachment and recurrent vitreous haemorrhage.
Risk factors
Risk factors for diabetic retinopathy include:
- Length of exposure to hyperglycaemia: the more time a patient’s eye has been exposed to high blood sugars, the greater the severity of DR.
- Duration since diabetes diagnosis: patients who have had diabetes for a greater length of time are more likely to develop DR.
- Hypertension: uncontrolled blood pressure can increase the risk of DR and its progression.
- Ethnicity: individuals with type 2 diabetes belonging to ethnic minority groups are more susceptible to developing DR compared to those of Caucasian background.
- Renal disease: diabetic nephropathy is another microvascular complication of diabetes, characterised by proteinuria. If present, it is a strong indicator that the patient will also have diabetic retinopathy.
- Pregnancy: has been shown to increase the rate at which DR progresses in several scenarios, including in severe baseline DR or concurrent hypertension (whether this is pre-existing or pregnancy-induced).
- Rapid improvement of blood sugar levels: rapidly lowering blood sugar levels (decrease of HbA1c >30mmol/mol or 3%) can increase the progression of DR.
- Hyperlipidaemia/hypercholesterolaemia
Clinical features
History
Many patients will remain asymptomatic, even in the presence of proliferative disease. Unfortunately, a lot of these patients will only present after developing clinically significant macular oedema (CSMO) or having a large vitreous haemorrhage.
Early disease may be incidentally picked up during regular eye tests.
In cases where patients develop symptoms, typical symptoms of diabetic retinopathy may include:
- Floaters: the result of small haemorrhages obscuring areas of vision and usually self-resolving.
- Blurred vision and distortion: central vision may be blurred if the macula is affected.
- Decreased visual acuity: gradual, painless reduction in the quality of vision.
- Loss of vision: a severe haemorrhage can result in a sudden complete and painless loss of vision.
- Blindness: a culmination of the disease if left untreated and uncontrolled.
Clinical examination
Visual acuity (VA):
- Currently, the Baily-Lovie chart (LogMAR chart) is most commonly used to test VA (Snellen chart has also been widely used previously).
- See our visual assessment guide for more details.
Fundoscopy:
- The fundus needs to be visualised to examine and monitor any changes that may occur as a result of retinopathy.
- The gold-standard for examination is with a slit lamp or through fundus photography.
- See our fundoscopy guide for more details.
Classification of diabetic retinopathy
DR is classified according to disease progression, which is determined by signs seen at the back of the eye.4
Diabetic retinopathy can be split into three classes:
- Non-proliferative
- Proliferative
- Diabetic macular oedema
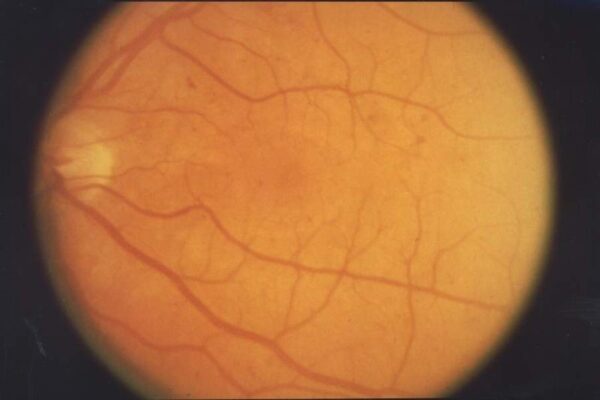
Non-proliferative diabetic retinopathy
Non-proliferative DR can be further subdivided into background retinopathy and pre-proliferative retinopathy.
Background retinopathy:
- The presence of at least one microaneurysm.
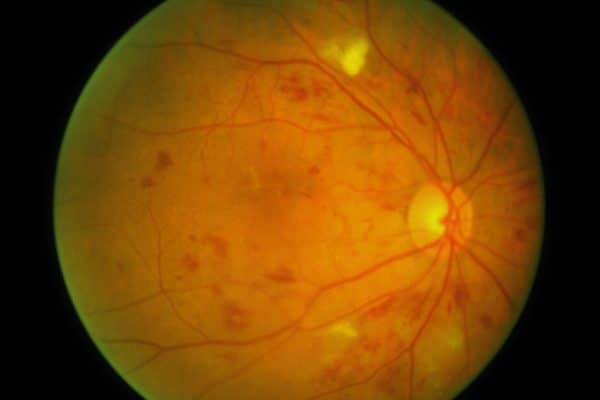
Pre-proliferative retinopathy:
- The presence of multiple microaneurysms with or without haemorrhages and hard exudates.
- Evidence of retinal ischaemia, for example, venous beading, arteriolar narrowing and intraretinal microvascular abnormalities (IRMAs).
- The severity of this stage depends on the number and size of clinical signs, and on how many quadrants of the retina are affected.5
The 4-2-1 rule of severe pre-proliferative diabetic retinopathy
- Blot haemorrhages in 4 quadrants
- Venous beading in 2 quadrants
- IRMA in 1 quadrant
Table 1. Signs of diabetic retinopathy.
| Sign | Description |
| Microaneurysms | “Out-pouching” results from weakened capillary walls. The earliest visible clinical sign of diabetic retinopathy. |
| Dot and blot haemorrhages | Damaged vessels may rupture and leak blood. |
| Hard exudates | Deposits of lipids that have leaked onto the retina through damaged vessels. |
| Cotton wool spots | Microinfarction of the retinal nerve fibre layer due to chronic ischaemia. |
| Venous beading | Venous changes are a reliable indicator of generalised ischaemia. |
| IRMAs | Intraretinal microvascular abnormalities are irregular formations of dilated capillary beds. |
Proliferative diabetic retinopathy
Proliferative diabetic retinopathy is characterised by new vessels on the disc (NVD) and/or new vessels elsewhere (NVE). It can also present as neovascular glaucoma, pre-retinal fibrosis and tractional detachment.
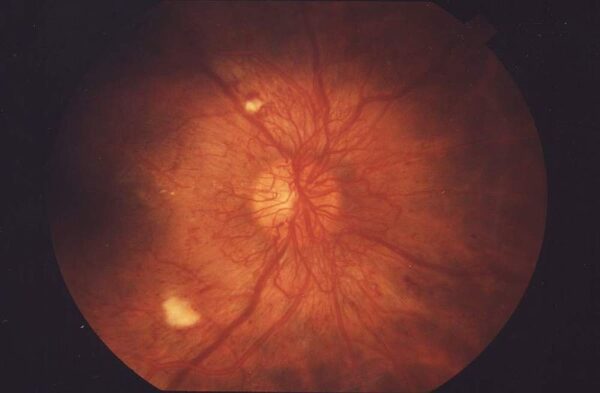
Table 2. Signs associated with proliferative diabetic retinopathy.
| Sign | Description |
| Neovascularization | Growth factors stimulate the formation of weak, leaky blood vessels because of chronic hypoxia. |
| Vitreous haemorrhage | Newly formed leaky vessels can extend into the vitreous humour which can then start to haemorrhage. |
| Retinal detachment | Typically tractional in aetiology. |
Diabetic macular oedema (DMO)
DMO is characterised by oedematous changes in or around the macula. As the macula is responsible for central vision, affected patients tend to complain of blurred vision when reading or difficulty recognising faces in front of them. DMO is the commonest cause of visual loss in patients with diabetes.9
DMO can be subcategorised into:
- Focal/diffuse macular oedema: the fluid that escapes from damaged vessels can be well-circumscribed (focal) or more widespread and poorly demarcated in nature (diffuse).
- Ischaemic maculopathy: patients will be symptomatic with defects in visual acuity due to ischaemia at the site of the macula. These areas are best visualised with fluorescein angiography.
- Clinically significant macular oedema (CSMO): CSMO describes significant changes associated with retinopathy, such as hard exudates and retinal thickening, found within a certain distance to the fovea or greater than a certain size.
Investigations
Laboratory investigations
HbA1c: to assess how well or poorly a patient’s diabetes is being controlled.
Optical coherence tomography (OCT)
OCT imaging provides a cross-sectional view of the retina.
It is more typically used when examining diabetic macular oedema to help quantify levels of oedema and retinal thickness in and around the macula.
Fluorescein angiography (FA)
FA is the gold-standard technique for visualising the vasculature of the retina.
It is used in DR for clearer identification of signs that may be difficult to see with clinical examination alone (e.g. retinal ischaemia and identifying microvasculature). FA can also highlight other retinal features such as microaneurysms and dot/blot haemorrhages.
Management
Many patients with DR will have little/no symptoms with DR and therefore require minimal intervention.
As with managing any medical condition, a benefit/risk analysis must be applied on an individual basis, with conservative methods attempted before considering more invasive options.
Medical management
Glycaemic control: ensuring blood sugar levels are well controlled will delay the progression of DR. Patients with diabetes should aim for a HbA1c between 48-58mmol/mol.
Blood pressure control: diabetic patients should aim to stabilise their blood pressure < 140/80mmHg to help prevent the progression of DR. Where the condition is already severe, patients should aim to keep their systolic < 130 mmHg.
Diet, exercise and smoking cessation: as part of managing diabetes, the patient should aim to make general lifestyle modifications to reduce the effect of diabetes.
Photocoagulation
Photocoagulation is the primary intervention in the management of proliferative DR and, in some cases, severe non-proliferative DR (especially if the patient is at high risk of progression e.g. pregnancy, frequent flyer, only one eye, pre-cataract surgery etc).
Photocoagulation involves using a laser is used to create numerous burns in the retina, thus destroying photoreceptors.10
With fewer photoreceptors, oxygen demand in the retina decreases and endothelial cells express fewer growth mediators (e.g. VEGF). Ultimately, the progression of DR is delayed.
There are two different methods to photocoagulation: focal/grid photocoagulation and pan-retinal photocoagulation (PRP).
Focal photocoagulation (FP)
In focal photocoagulation, a specific point of leakage is identified and targeted with the laser. Alternatively, grid photocoagulation targets more diffuse retinal thickening and oedema with no obvious point of leakage.
Complications of photocoagulation include the decreased quality of central vision and/or formation of a paracentral scotoma. In a minority of patients, DMO may worsen because of FP.
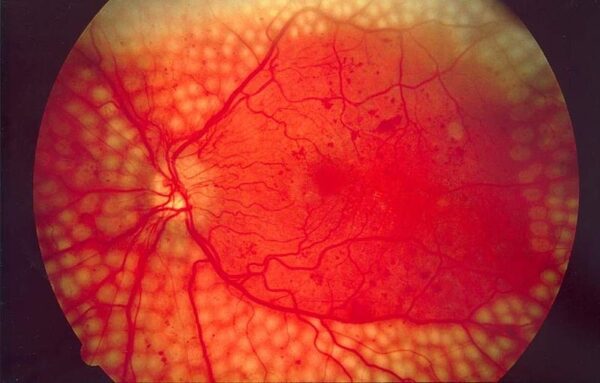
Pan-retinal photocoagulation (PRP)
The periphery of the retina is targeted with the aim of achieving a global reduction in oxygen demand. More commonly used than FP, approximately 1200-1500 burns can be made to the retina using this technique.
Complications of PRP include a restricted peripheral vision, reduced quality of night vision, ocular pain and, as with FP, there may be a worsening of macular oedema.
Intravitreal anti-VEGF/corticosteroid injections
Anti-VEGF injections focus on minimising neovascularization and thus are used in proliferative diabetic retinopathy. Aflibercept (Eylea) and Ranibizumab (Lucentis) are two commonly used anti-VEGF agents in the treatment of DR.
Although the mechanism of action is not completely understood, trials have shown intravitreal corticosteroids can also be effective in improving visual acuity and reducing maculopathy.
Corticosteroids may be used either as an adjunct to other management options or as primary therapy. Usually, patients will experience the full benefit of steroids after one week, with effects potentially remaining for up to 6 months.
Complications include cataract formation and increased intraocular pressure.
Anti-VEGF is contraindicated in patients who have suffered from a stroke or myocardial infarction in the last 3 months.
Vitrectomy
A potential complication of proliferative DR is bleeding into the vitreous humour, which further increases the risk of retinal detachment. In many cases, waiting for the haemorrhage to settle can allow sufficient view to perform laser therapy.
However, in persistent haemorrhage or in central, sight-threatening tractional retinal detachment a vitrectomy may be performed. This allows for the removal of the vitreous and repair of any scarring/detachment of the retina. Photocoagulation may be used intra-operatively to reduce the risk of further neovascularization post-operatively.
Screening
In the United Kingdom, the NHS provides an annual eye screening programme for any individual over the age of 12 with diabetes.12
The screening programme aims to promptly identify and manage any changes associated with diabetic retinopathy.
Complications
Neovascular glaucoma13
Diabetic retinopathy is one of several causes of neovascular glaucoma, a type of secondary glaucoma.
Neovascularization can occur within the iris and its trabecular meshwork (rubeosis) causing a narrowing and closure of the drainage angle and therefore increased intraocular pressure.
The typical presentation may include a patient complaining of an acutely painful, red eye. The patient may also complain of vision loss.
As well as managing underlying diabetes and associated retinopathy, neovascular glaucoma may also be directly managed medically, with laser therapy or surgically.
Other complications
Retinal detachment and vitreous haemorrhage are other complications of diabetic retinopathy, as discussed earlier.
Prognosis
If left untreated, approximately 50% of patients with proliferative DR will lose their vision in 2 years.
Around 90% of affected patients will have lost most of their vision within 10 years.
Those with proliferative DR that undergo treatment can reduce their risk of severe vision loss by 50%.
Key points
- Diabetic retinopathy is characterised by damage to the retina because of diabetes.
- Chronically elevated levels of glucose damage the microvasculature supplying the retina, which compromises oxygen supply and leads to injury of retinal cells.
- It is a sight-threatening condition if not managed appropriately.
- Diabetic retinopathy is classified as either non-proliferative or proliferative.
- The term ‘diabetic macular oedema’ describes ischaemic and oedematous changes at the macula.
- Many patients remain asymptomatic, even in the presence of proliferative disease.
- Photocoagulation is the mainstay of active treatment, with intravitreal VEGF injections also having an important role in the management of proliferative disease.
- Complications of diabetic retinopathy include neovascular glaucoma, retinal detachment, and vitreous haemorrhage.
Reviewer
Mr George Moussa
ST6 Ophthalmology Trainee
Editor
Dr Chris Jefferies
References
- Wang, W; Yo, A. Diabetic Retinopathy: Pathophysiology and Treatments. 2018. Available from: [LINK]
- Diabetes.co.uk. Diabetes Prevalance. 2019. Available from: [LINK]
- Tidy, C. Diabetic Retinopathy and Diabetic Eye Problems. 2016. Available from: [LINK]
- The Royal College of Ophthalmologists. Diabetic Retinopathy Guidelines. 2012. Available from: [LINK]
- Wu et. al. Classification of diabetic retinopathy and diabetic macular edema. 2013. Available from: [LINK]
- National Eye Institute. Background retinopathy. License: [Public domain]. Available from [LINK]
- Community Eye Health Journal. Pre-proliferative diabetic retinopathy. License: [CC BY-NC 2.0]. Available from: [LINK]
- National Eye Institute. Proliferative Retinopathy. License: [Public domain]. Available from [LINK]
- Somilleda-Ventura, S.A., Razo Blanco-Hernández, D.M., Serafín-Solís, S. et al. Should the outcome of focal photocoagulation for center-sparing diabetic macular edema require expanding the definition of center involvement?. Available from: [LINK]
- Stefánsson, E. The Mechanism of Retinal Photocoagulation – How Does the Laser Work? 2008. Available from: [LINK]
- National Eye Institute. Pan-retinal photocoagulation. License: [Public domain]. Available from: [LINK]
- nhs.co.uk. Overview – Diabetic Retinopathy. 2018. Available from: [LINK]
- Bright Focus Foundation. What is Neovascular Glaucoma? 2017. Available from: [LINK]