- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Haematinics are the nutrients needed by the bone marrow to make blood cells in the process of haematopoiesis. Without adequate amounts of these nutrients, cytopenia(s) and related symptoms can develop. Excess amounts can also be pathological and can point to various underlying disease states.
This guide to haematinics interpretation will focus on deficiencies of the most clinically relevant haematinics: vitamin B12 (cobalamin), vitamin B9 (folate), and iron.
Other haematinics not covered in this guide include:
- Vitamin A
- Vitamin B2 (riboflavin)
- Vitamin B3 (nicotinic acid)
- Vitamin B6
- Vitamin C
- Vitamin E
- Copper
- Cobalt
It is essential to consider the history and examination findings when interpreting haematinics. The relevance of results can change depending on the patient context and should be interpreted alongside a thorough clinical assessment.
Iron
Investigating a patient’s iron status can become complicated as no single test accurately represents all stages of iron metabolism and some of the available tests can be affected by inflammation and renal disease.
Given how common iron deficiency anaemia is, it is important to understand the tests used to investigate iron deficiency and how to interpret results correctly.
When to investigate
Iron deficient patients can develop microcytic anaemia and may have a range of clinical features.
Most of these symptoms are related to anaemia, such as fatigue, shortness of breath, weakness and reduced exercise tolerance. Other symptoms can include pica (e.g. ice craving) and restless leg syndrome.
Typical clinical findings of iron-deficiency anaemia on examination may include:
- General and conjunctival pallor
- Atrophic glossitis
- Angular cheilitis
- Koilonychia (spoon-shaped nails) – less common
Initial screening investigations
If iron deficiency anaemia is suspected, initial screening investigations should include:
- Full blood count (FBC)
- C-reactive protein (CRP)
- Serum ferritin
Full blood count (FBC) and red cell parameters
These tests allow the identification and quantification of anaemia by measuring the haemoglobin (Hb) and haematocrit (Hct) which is typically seen in iron deficiency. Although these measures do not provide any direct information about iron stores.
To gain more information about iron status, review the mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC). These may be low in iron deficiency, while red cell distribution width (RDW) may be elevated.
These findings are non-specific and may not be present in all iron-deficient patients.
For more information, see the Geeky Medics guide to full blood count interpretation.
Serum ferritin
Ferritin is an intracellular protein complex that binds iron and is responsible for most iron storage in the body.
Small amounts of ferritin are released into the serum to transport and absorb excess iron and therefore act as a surrogate serum marker for body iron storage.
There is a direct correlation between serum ferritin levels and overall iron stores in health. However, serum ferritin is an acute-phase protein, and so increases in inflammatory states, chronic kidney disease, liver disease, and malignancy.
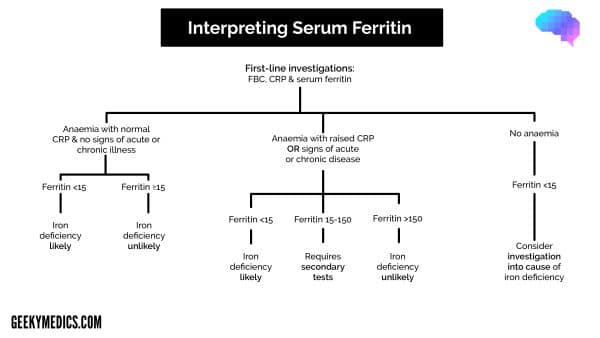
The British Society for Haematology (BSH) guidelines suggest a serum ferritin level of <15 μg/l is indicative of an iron deficiency in those aged >5 years.
A level of <150 μg/l should act as a trigger to consider further investigations for potential iron deficiency if a patient has a concurrent inflammatory condition (acute or chronic) or renal impairment.
Therefore, CRP is suggested as part of the initial screening test, as serum ferritin within the normal range cannot exclude iron deficiency if a patient has raised inflammatory markers or a history of acute or chronic illness.
Secondary investigations
If the first screening tests are inconclusive, but the suspicion remains that a patient might be iron deficient, BSH suggest requesting secondary investigations which are listed below.
Transferrin saturations
Transferrin saturation is the ratio of total serum iron (or the total iron-binding capacity) to transferrin expressed as a percentage.
Transferrin is the primary serum iron transporter molecule in the body. In an iron-deficient state, the body produces more transferrin to increase the total iron-binding capacity and so acquire more iron for its cells.
There is no strong evidence for a diagnostic threshold, but the BSH consensus guidelines suggest levels <16% as supportive of a diagnosis of iron deficiency if initial tests are inconclusive.
Blood film
A blood film can be helpful if there is diagnostic uncertainty and can show morphological changes, which might support the conclusion that a patient is iron deficient.
Blood film features which make iron deficiency more likely include anisocytosis, microcytosis, hypochromia, pencil cells, target cells, and elliptocytes.
Advanced red cell parameters
These are available on modern analysers and most UK laboratories would be able to run these tests relatively easily. Different analysers will run slightly different tests but they include mean reticulocyte haemoglobin concentration, percentage of red cells that are hypochromic, and reticulocyte haemoglobin equivalent.
Given that they are relatively easy to perform, BSH suggest they can be used as part of secondary testing. Mean reticulocyte haemoglobin of <29 pg can be supportive of a diagnosis of iron deficiency and may be useful in predicting iron response in patients with chronic kidney disease.
Other investigations of iron metabolism
These are not usually required to diagnose iron-deficiency anaemia but are available from most laboratories and can support the diagnosis. Below is a list of the more common investigations.
Serum iron
Serum iron only measures a fraction of the iron in the blood. It can only measure the ferric form (Fe3+) and not the iron incorporated in haemoglobin molecules.
Serum iron levels show diurnal variation and are sensitive to recent iron intake therefore, measuring serum iron in isolation has no role in determining a patient’s iron stores.
Total iron-binding capacity (TIBC)
The TIBC is calculated by taking a serum sample and adding excess iron to fully saturate the iron carrying molecules.
The TIBC is a measurement of the total iron concentration in the sample when fully saturated.
TIBC can rise in an iron-deficient state, but specificity is poor. Hence, BSH does not recommend routinely using this to assess iron stores.
Transferrin
Transferrin is the main serum iron transporter molecule which can be measured in a patient’s serum.
Like TIBC, transferrin can rise in iron deficiency as the body tries to increase the total iron-binding capacity. However, transferrin is a negative acute-phase protein and so decreases in inflammatory states.
Soluble transferrin receptor (STFR)
Transferrin receptors are present on developing red cells and can be detected in their soluble form in peripheral blood.
In iron deficiency, these developing cells display an increased number of transferrin receptors as they try to acquire more iron, this increase can be measured in the serum.
Unlike ferritin, this is not affected by inflammation and so can be helpful in distinguishing anaemia of chronic disease from iron deficiency anaemia.
A rise in STFR is not specific to iron deficiency with other potential causes including haemolysis, thalassaemia, megaloblastic anaemia, and hypoxia. STFR can also be normal in mild deficiency, only becoming abnormal in more advanced stages.
Due to the lack of specificity and comparatively high cost, BSH does not recommend measuring STFR when investigating iron deficiency.
Summary table
Table 1. Interpreting iron studies.
|
|
Iron deficiency anaemia | Anaemia of chronic disease | Acute-phase response |
|
Ferritin |
↓/Normal |
Normal |
↑↑ |
|
Transferrin/TIBC |
↑ |
Normal/↓ |
↓↓ |
|
Transferrin saturations |
↓ |
Normal/↓ |
– |
|
STFR |
↑/Normal |
Normal |
– |
Treatment of iron deficiency anaemia
For most patients, oral iron is a safe, effective and cheap method of treating iron deficiency. Co-administration of vitamin C improves the absorption of oral iron.
Traditional dosing guidelines suggested aiming for 100-200mg of elemental iron a day however, more recent studies performed in iron deficient, non-pregnant women have shown that once-daily dosing of around 45-80mg elemental iron is more effective and avoids many of the gastrointestinal side effects. This amount of elemental iron roughly equates to one tablet of either ferrous fumarate 210mg or ferrous sulphate 200mg. A response in the haemoglobin count should be seen within a few weeks of starting therapy.
In certain scenarios, intravenous iron may be preferred. This includes patients with chronic kidney disease, inflammatory bowel disease, those intolerant of oral iron, those needing rapid replacement due to the degree of anaemia or certain pregnant patients. The safety profile has improved with more recent formulations but there is still a risk of anaphylactoid reactions and permanent skin staining.
Vitamin B12
Testing for a patient’s vitamin B12 and folate status is challenging as existing investigations are not sensitive or specific and show wide variability between different laboratories.
For this reason, routine screening is not indicated for any patient group. Instead, investigations should be performed in response to specific clinical indicators.
As vitamin B12 and folate share a close relationship in human metabolism and so present with similar features, they should be investigated simultaneously.
Clinical features of vitamin B12 deficiency
B12 deficiency often develops over years, with clinical features occurring insidiously.
Patients with B12 deficiency can present with features related to anaemia such as fatigue, shortness of breath, weakness, and reduced exercise tolerance as well as neuropsychological features (motor and sensory peripheral neuropathies, ataxia, retinopathy, optic atrophy, cognitive impairment etc.) and glossitis.
In severe deficiency, subacute degeneration of the spinal cord can develop.
Importantly, not all patients with clinical vitamin B12 deficiency will have anaemia or macrocytosis.
Therefore, if a patient presents with clinical features of B12 deficiency, is in an at-risk population (see below) or has been identified to have macrocytic anaemia, investigations of B12 stores should be undertaken.
Infants can also present with faltering growth, movement disorders or developmental delay.
Causes of vitamin B12 deficiency
Causes of vitamin B12 deficiency include:
- Malabsorption: pernicious anaemia, Crohn’s, coeliac disease, gastric/bariatric surgery, atrophic gastritis
- Dietary: vegetarians/vegans
- Infection: H.pylori, giardia, tapeworm
- Infants: congenital causes
Specific scenarios
Medications
Certain medications can affect serum B12 assays creating misleading results. However, they rarely lead to clinically significant deficiencies, and B12 levels should only be checked in the presence of objective features of deficiency.
Metformin, anti-convulsants, proton pump inhibitors/H2 antagonists, hormone replacement therapy/combined oral contraceptive pill, colchicine and certain antibiotics can all decrease serum B12 levels.
Gastrointestinal surgery
Patients who have undergone gastric surgery or bariatric surgery can often develop vitamin B12 deficiency.
These patients should undergo monitoring of B12 levels and receive advice on adopting a diet rich in B12 (red meat, salmon, milk, cheese etc.). Intramuscular replacement should be commenced if levels are falling despite this.
Pregnancy
A physiological reduction in serum B12 levels occurs in up to 30% of individuals by the third trimester of pregnancy, complicating assessment. If in doubt about the relevance of results, seek specialist advice.
Multiple myeloma
The presence of paraproteins can reduce serum B12, but rarely reflects clinical deficiency.
Primary testing
Serum cobalamin is the primary investigation suggested by BSH and should only be requested in the presence of objective clinical markers of deficiency. When investigating a patient for vitamin B12 deficiency, the initial blood tests should include serum cobalamin, full blood count, and a blood film.
Serum cobalamin
The most common investigation in the UK for vitamin B12 is serum cobalamin, but levels do not always correlate clinically.
It is possible to be clinically deficient in vitamin B12 with a normal range serum cobalamin level and vice versa.
False normal results can occur in the presence of very high titres of anti-intrinsic factor antibodies in pernicious anaemia. Falsely low serum levels may occur in concurrent folate deficiency or conditions listed above in the specific scenarios section.
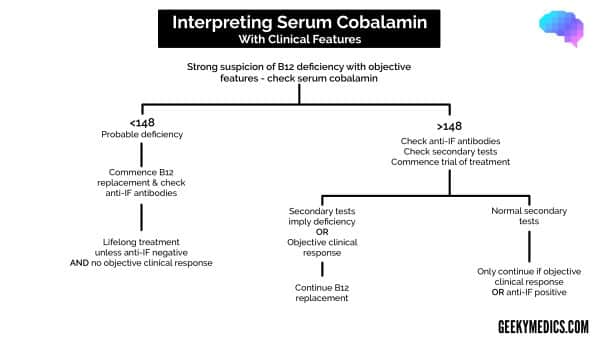
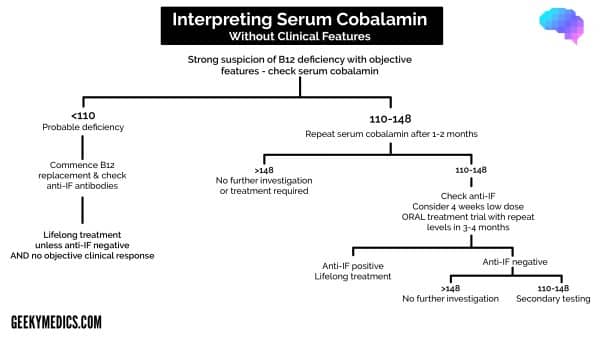
While there is no agreed normal level, most UK labs use a cut-off of <148pmol/l as evidence of B12 deficiency if objective clinical features are present.
BSH guidelines differentiate between patients with and without objective clinical features of B12 deficiency when interpreting these results (see flowcharts below).
Full blood count (FBC) and red cell parameters
Features which might suggest a vitamin B12 deficiency include anaemia and macrocytosis.
However, it is possible to be B12 deficient with a normal full blood count. Reticulocyte counts will be low or normal as the bone marrow cannot make new cells without vitamin B12.
Blood film
A blood film can help establish the diagnosis and assess for concurrent diseases. Features on blood film are non-specific, but can include macrocytosis, hypersegmented neutrophils and oval macrocytes.
Investigating the cause
If a patient with B12 deficiency has a family history of pernicious anaemia or a personal history of an autoimmune condition, it is important to investigate for pernicious anaemia. BSH suggest screening for pernicious anaemia in patients with both clinical features and laboratory confirmed B12 deficiency.
Anti-intrinsic factor antibodies (Anti-IF)
Anti-intrinsic factor antibodies are found in pernicious anaemia and have a low false-positive rate with a high positive predictive value. They are present in 40-60% of pernicious anaemia cases. False-positive results can occur following recent B12 injections.
Anti-gastric parietal cell antibodies
Anti-gastric parietal cell antibodies are less specific, being found in 10% of the background population who do not have pernicious anaemia. However, they are found in up to 80% of patients with the disease.
Summary charts of BSH guidance
Secondary testing
Depending on the clinical presentation, secondary testing may be required. Secondary testing is indicated if a patient presents with convincing clinical features of vitamin B12 deficiency (e.g. macrocytic anaemia and glossitis or neurological symptoms) but has normal serum cobalamin levels.
Secondary testing for B12 deficiency includes:
- Total plasma homocysteine: raised in B12 deficiency but can also be elevated in folate deficiency, B6 deficiency, renal disease, and hypothyroidism.
- Plasma methylmalonic acid: raised in B12 deficiency and in renal disease, haemoconcentration and small bowel overgrowth.
- Holotranscobalamin: more sensitive but not available in all labs. It is low in vitamin B12 deficiency.
Table 2. Interpreting secondary tests for B12 deficiency.
|
|
B12 deficiency | Folate deficiency |
|
Plasma homocysteine |
↑ |
↑ |
|
Plasm methylmalonic acid |
↑ |
– |
|
Holotranscobalamin |
↓ |
– |
Treatment of B12 deficiency
In the United Kingdom, guidelines for the treatment of B12 deficiency suggest using intramuscular (IM) vitamin B12 injections with the dosing and frequency depending on symptoms and response:
- If no neurological symptoms: 1mg IM three doses a week for 2 weeks, then every 3 months
- If neurological symptoms present: 1mg IM alternate days until no further improvement in symptoms (minimum 3 weeks), then every 2 months
Once treatment is commenced, an increase in reticulocyte count should occur in the first 7-10 days.
Folate
Folate is the term for all biological forms of vitamin B9, while folic acid refers to the synthetic form used for treatment.
Both forms are absorbed in the terminal ileum, and half of the body’s folate is found in the liver. It is often measured simultaneously with vitamin B12 as they share a close relationship in human metabolism with similar clinical features in deficiency.
As folate is important in DNA synthesis, the rapidly dividing cells of the body are those first affected.
Symptoms of folate deficiency can include those related to anaemia (shortness of breath, fatigue, weakness etc.), and mouth ulcers/glossitis. Therefore, folate levels are generally measured in similar scenarios to vitamin B12.
An isolated folate deficiency is rare, if a patient is deficient in both folate and vitamin B12 they should receive B12 replacement before initiating folate replacement due to the risk of precipitating subacute degeneration of the spinal cord.
Causes of folate deficiency
Causes of folate deficiency can be categorised into conditions that either inhibit absorption, increase secretion or those increasing the body’s requirements for folate:
- Dietary: diet deficient in folate-rich foods (legumes, broccoli, asparagus, brown rice, folate fortified foods)
- Alcoholism: alcohol intake >80g/10 units a day
- Gastrointestinal disorders: coeliac or any disorder affecting the small bowel
- Pregnancy: preferential delivery of folate to foetus rather than mother’s tissues, deficiency in utero increases the risk of neural tube defects
- Haematological disorders: increased red cell destruction (sickle cell anaemia, haemolytic anaemias) and those with abnormal haematopoiesis
- Exfoliative skin disorders (e.g. psoriasis)
- Medications: anti-epileptics
Primary testing
If folate deficiency is suspected, the initial tests should include a serum folate level and a full blood count.
Serum folate
The serum folate level is the most useful initial screening test. No internationally recognised level defines deficiency, but a level <7nmol/l is generally used. Below this level, the risk of megaloblastic anaemia sharply increases.
Therefore, there is a grey area from 7-10nmol/l, in which case a treatment trial may be helpful.
Serum folate is not a perfect test as several conditions can cause false results.
Falsely low levels can occur in anorexia, acute alcohol intake, pregnancy, anti-epileptic therapy, and post-haemodialysis. Falsely high levels can occur if the test is performed too soon after oral folate ingestion.
Full blood count/blood film
The development of anaemia due to folate deficiency is a late consequence, the lack of folate affects developing red cells and leads to the presence of large, abnormal red cells called megaloblasts in peripheral blood. This causes a raised MCV on the full blood count.
Secondary testing
If the clinical suspicion of folate deficiency is high but serum folate is in the normal range, BSH guidelines suggest considering secondary testing. These can include:
- Red cell folate: lots of laboratory variables limiting use, time-consuming and expensive
- Plasma total homocysteine: high in folate deficiency but non-specific as can be raised in B12 deficiency, B6 deficiency, renal disease and hypothyroidism
Treatment of folate deficiency
If a patient is folate deficient, it is important to assess for concurrent vitamin B12 deficiency.
If a patient is deficient in both folate and B12, they should be started on B12 replacement first to avoid precipitating or worsening neurological features of B12 deficiency.
The usual treatment of folate deficiency is folic acid 5mg once daily for 3-4 months unless the cause of the folate deficiency is chronic (e.g. haemolysis) in which case a longer duration of treatment might be required.
Reviewer
Dr Tom Fail
Haematology SpR
Editor
Dr Chris Jefferies
References
- Fletcher, A., Forbes, A., Svenson, N., Wayne Thomas, D. (2022), Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br J Haematol, 196: 523-529.
- Devalia, V., Hamilton, M.S., Molloy, A.M. and (2014), Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol, 166: 496-513.
- Camaschella C. (2015) Iron-deficiency anemia. New England journal of medicine. May 7;372(19):1832-43.