- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
The spinal cord is a conduit for sensory nerves travelling toward the brain and motor nerves travelling from the brain.
In this article, we will discuss the differences between grey and white matter of the spinal cord. We’ll also cover the protective coating of the spinal cord and the blood vessels of the spinal cord. More information on the tracts of the spinal cord can be found in the ascending and descending tract articles.
Anatomy of the spinal cord
The spinal cord is a cylinder that is roughly 45 cm long and 1 cm wide.
It extends from the external margin of the foramen magnum as a continuation of the medulla oblongata, down to the L2 vertebral level, and is entirely housed in the spinal meningeal layers.
As the spinal cord transitions through the cervical and lumbar regions, there are two ‘enlargements.’
These enlargements reflect the high number of lower motor nuclei present in these regions. The cervical enlargement is for the brachial plexus and lies from C4/5 to T1.
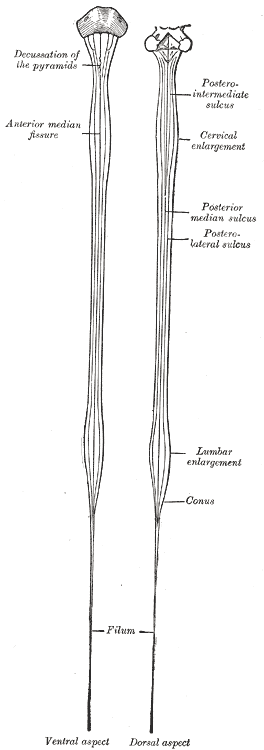
The lumbar enlargement is for the lumbosacral plexus and lies from T11 to L1/2.
As the spinal cord reaches L1, it turns into a conical structure known as the conus medullaris (medullary cone).
The conus medullaris is short and at L2, sends off the remaining spinal nerves of L2-Co1. This bundle of nerves is known as the cauda equina (the horse’s tail).
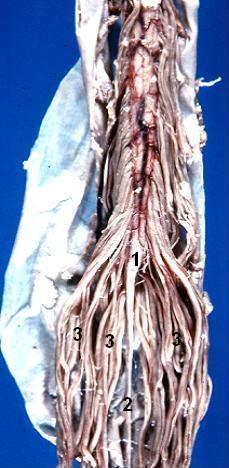
Clinical relevance: cauda equina syndrome
Cauda equina syndrome refers to a distinct set of symptoms relating to impingement of the cauda equina. Cauda equina syndrome involves the compression of the nerves in the cauda equina, which often occurs secondary to vertebral disc prolapse or malignancy.
Cauda equina syndrome is a medical emergency, and time is of the essence in recognising and treating this pathology. The symptoms of cauda equina syndrome are ‘saddle’ anaesthesia, loss of urinary and anal sphincter tone and lower back pain.
Saddle anaesthesia refers to a loss of sensation on the proximal medial thigh and inguinoscrotal and anal areas.
The gold standard for diagnosis is MRI, which will reveal compression of the spinal nerves (+ conus medullaris compression).
Dexamethasone is given to temporise inflammation, and definitive treatment is completed with radiation or surgery followed by radiation.
The prognosis heavily depends on the aetiology and ambulatory status at presentation – patients who ambulate at presentation generally have a much better prognosis.
For more information, see the Geeky Medics guide to cauda equina syndrome.
Spinal cord transverse section
When looking at a transverse section of the spinal cord, it is important to assess the orientation. There are several key features that tell us which way the spinal cord is facing. These structures are:
- Ventral median fissure
- Dorsal median sulcus
- Dorsal root ganglion (if included in section)
- Ventral horn
- Dorsal horn
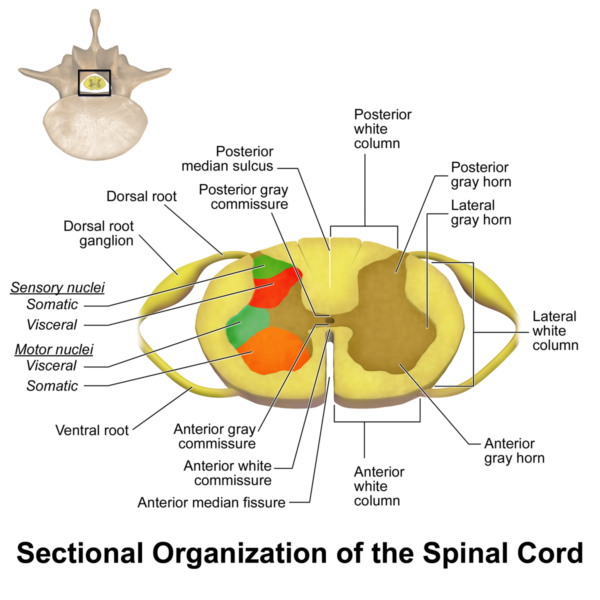
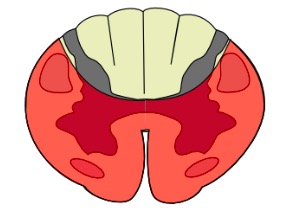
The ventral median fissure is an invagination of the spinal cord located on the ventral (anterior) surface. It helps in separating the left and right halves of the spinal cord.
The dorsal median sulcus is analogous to the ventral median fissure and is located dorsally (posteriorly) on the spinal cord. It separates the left and right dorsal columns of the spinal cord. Lateral to the dorsal median sulcus are the paired dorsolateral sulci, which are important sites for the entry of sensory afferents into the spinal cord.
Within the spinal cord, there is both grey and white matter:
- Grey matter contains a high density of neuron cell bodies and low density of axonal tracts.
- White matter contains a high density of myelinated axonal tracts and low density of neuron cell bodies.
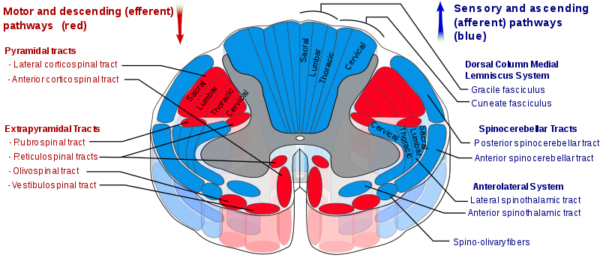
The white matter is separated into tracts and fasciculi discussed in the spinal cord ascending and descending tract articles.
The grey matter is divided into two general regions:
- Ventral horn: containing motor nerve cells
- Dorsal horn: containing sensory nerve cells
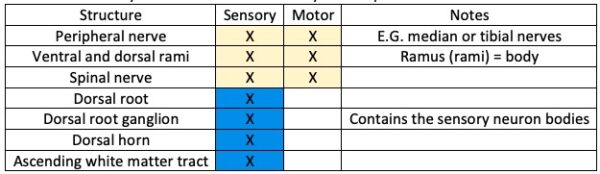
The dorsal and ventral horns give rise to the dorsal and ventral roots, respectively. The dorsal and ventral roots converge at the intervertebral foramina and form the spinal nerve.
As the spinal nerve leaves the intervertebral foramina, it diverges into the dorsal and ventral rami.
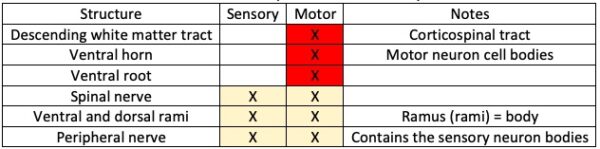
Figure 4 and 5 show that the spinal cord roots and horns are specific to either sensory (dorsal) or motor (ventral), but structures further away from the spinal cord carry both sensory and motor information.
The flow of sensory information from the body to the spinal cord is as follows (correlating with the colour of the tracts in the image below):
The flow of motor information from the spinal cord to the body is as follows (correlating with the colour of the tracts in the image below):
Spinal meningeal layers
As the spinal cord and brain are parts of the central nervous system, they are both protected by the meningeal layers.
There are three meningeal layers, from superficial to deep:
Dura mater (“tough mother”)
A tough, thick opaque layer of connective tissue which extends from the foramen magnum to the filum terminale. It is separated from the osseous vertebral canal by the epidural space.
The epidural space contains loose areolar connective tissue and the internal vertebral venous plexus.
Arachnoid mater (“spider-like mother”)
Separated from the dura mater by the subdural space and separated from the pia mater by the subarachnoid space.
The subarachnoid space contains cerebrospinal fluid (CSF). There is a large lumbar cistern of subarachnoid space filled with CSF around the conus medullaris and cauda equina, this is the target site for lumbar punctures.
Pia mater (“tender mother”)
The pia mater adheres to the spinal cord, including the ventral median fissure and dorsal median sulcus. It extends ~20cm to the coccyx as the filum terminale, which picks up a layer of dura mater that surrounds and strengthens it. The filum terminale anchors the spinal cord within the subarachnoid space.
Blood vessels
Arteries
Three longitudinal arteries supply the spinal cord with blood: the paired posterior spinal arteries and an anterior spinal artery.
The anterior spinal artery is derived from the two vertebral arteries. It lies in the ventral median fissure and supplies the anterior two-thirds of the spinal cord, anterior to the dorsolateral sulci.
The posterior spinal arteries are derived from the posterior inferior cerebellar arteries (75%) or the vertebral arteries (25%). They lie in the dorsolateral sulci and supply the posterior third of the spinal cord, between the dorsolateral sulci
These three vessels are fed by the anterior and posterior segmental medullary arteries (also called the radicular arteries). The anterior segmental medullary artery can feed the anterior spinal artery, the largest of which is the artery of Adamkiewicz.
The Artery of Adamkiewicz is derived from the inferior intercostal and lumbar arteries and supplies the inferior two-thirds of the spinal cord.
The posterior segmental medullary arteries enter the spinal cord through the dorsolateral sulci to supply deep spinal cord structures.
Veins
Each of the arteries mentioned above has a paired vein:
- Anterior spinal vein
- Paired posterior spinal veins
These veins contain no valves and lie within the same fissures and sulci as their arterial counterparts.
The segmental medullary (radicular) veins drain into these three vessels. The anterior and posterior spinal veins drain into a pair of larger anterior, and a pair of smaller posterior internal vertebral venous plexuses.
These then drain segmentally into deep cervical, azygous and lumbar veins.
Clinical relevance: anterior spinal artery syndrome
Anterior spinal artery (ASA) syndrome is often described as an incomplete spinal cord lesion that involves loss of arterial supply to the ventral two-thirds of the spinal cord.
Among the many aetiologies, more commonly it can be caused by thrombus formation, embolic disease and aortic dissection. One of the more commonly affected arteries is the artery of Adamkiewicz.
The onset of ASA syndrome is typically sudden and painless. Lower motor neuron symptoms are apparent below the level of the lesion. Pain and temperature at or just below the level of the lesion (depending on the level of decussation). Autonomic dysfunction may manifest with erectile dysfunction, hypotension and bowel and bladder dysfunction due to the loss of sympathetic outflow.
Light touch, vibration and joint proprioception are typically spared, as they are contained within the dorsal columns.
MRI is the gold standard for spinal cord imaging and should be completed in all patients with suspected ASA syndrome.
ASA syndrome carries a poor prognosis, with a mortality rate of up to 30%. Most patients with ASA syndrome are unlikely to recover given the poor regenerative capacity of neural tissue.
References
- Henry Vandyke Carter. License: [Public domain]
- John A Beal, PhD Dep’t. of Cellular Biology & Anatomy, Louisiana State University Health Sciences Center Shreveport. License: [CC BY]
- BruceBlaus. License: [CC BY-SA]
- Polarlys and Mikael Häggström. License: [CC BY-SA]
- Niels Olson. License: [Public domain]