- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Haemolysis is the premature destruction of red blood cells (RBCs) before their typical lifespan of 120 days.1
Haemolytic anaemia results when the bone marrow cannot sufficiently compensate for the loss of red cells, leading to a fall in red cell count and haemoglobin concentration.
Aetiology
Pathophysiology
Normal RBCs have a lifespan of around 120 days, after which they are destroyed by macrophages in the reticuloendothelial system (mainly the spleen). RBCs are continuously replenished through erythropoiesis, which in adults occurs in the bone marrow under the influence of the cytokine erythropoietin.2
Erythropoietin is released by the kidney in response to hypoxia. It binds to receptors on RBC precursors and stimulates their differentiation to mature erythrocytes, which are released from the marrow into the blood.
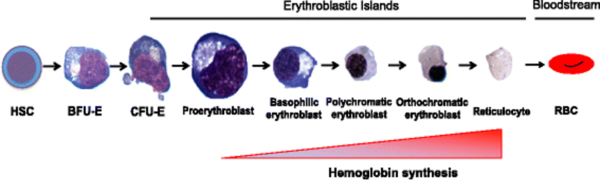
In haemolytic anaemia red cells are destroyed prematurely, either within the circulation (intravascular haemolysis) or within the reticuloendothelial system (extravascular haemolysis).
The fall in the RBC count leads to a reduced oxygen-carrying capacity of the blood and relative hypoxia, which stimulates EPO secretion, upregulating erythropoiesis.2
This results in an increase in the reticulocyte count (reticulocytosis), a sign that the marrow is attempting to compensate for a loss of red cells.
If the bone marrow is not able to sufficiently compensate, the haemoglobin concentration falls and anaemia results.
Intravascular vs extravascular haemolysis
Haemolysis can occur in two locations. Extravascular haemolysis occurs primarily in the spleen. It is more common than intravascular haemolysis.
Intravascular haemolysis is the breakdown of red blood cells within the circulation, leading to the release of free haemoglobin into the blood. It is less common than extravascular haemolysis.
In clinical practice, the distinction between intravascular and extravascular haemolysis is not clear cut and there may be significant overlap between the two.
Causes of haemolytic anaemia
There are many conditions that can lead to haemolysis. Conditions leading to intravascular haemolysis include:1
- Complement activation leading to intravascular lysis of RBCs. This can occur in ABO mismatched transfusions, severe cases of autoimmune haemolytic anaemia (AIHA) and rarer diseases such as paroxysmal nocturnal haemoglobinuria (PNH) and paroxysmal cold haemoglobinuria (PCH).
- Direct cellular destruction by toxins, trauma or lysis. Insect or snake venom and certain infections such as malaria and clostridial pathogens may cause direct cellular destruction. Freshwater drowning can lead to red cell lysis due to osmotic forces. Traumatic causes include extracorporeal circuits (e.g. ECMO, haemodialysis), mechanical heart valves, aortic stenosis and prolonged marching.
- Microangiopathic haemolytic anaemia (MAHA) occurs when abnormalities of the small blood vessels lead to shearing forces and intravascular rupture of RBCs. Conditions in which MAHA occur include disseminated intravascular coagulation (DIC), thrombotic thrombocytopenic purpura (TTP), haemolytic uraemic syndrome (HUS) and haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome – which are collectively referred to as the thrombotic microangiopathies.
- Oxidative haemolysis occurs where the protective mechanisms of RBCs are overwhelmed. For example, in G6PD and pyruvate kinase deficiency.
The primary mechanism of extravascular haemolysis is through phagocytosis of RBCs within the spleen. This can occur due to:1
- Antibody binding leading to opsonization and phagocytosis of tagged RBCs within the reticuloendothelial system. Examples of conditions in which this occurs include haemolytic disease of the fetus and newborn (HDFN), delayed haemolytic transfusion reactions and autoimmune haemolytic anaemia (although, as stated above in severe cases of AIHA intravascular complement-mediated lysis can occur).
- Infection and toxins which are detected by spleen-resident macrophages leading to RBC phagocytosis.
- Intrinsic red cell defects such as haemoglobinopathies and membrane disorders (e.g. hereditary spherocytosis and elliptocytosis). These cause the RBC to become trapped within the spleen where they are destroyed.
- Splenomegaly/hypersplenism due to a variety of secondary causes. For instance, myeloproliferative disorders such as chronic myeloid leukaemia (CML) or hypersplenism secondary to portal hypertension caused by cirrhosis.
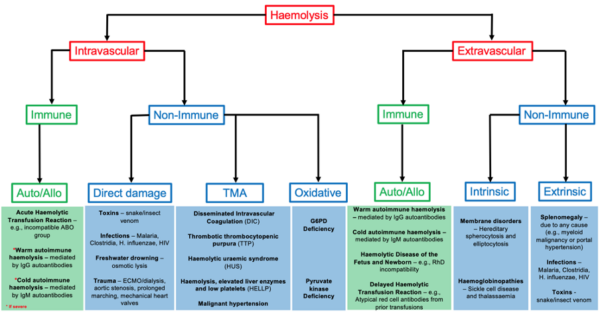
A summary of the causes of haemolysis is shown in Figure 2.3
Clinical features
History
Typical symptoms of anaemia may include:
- Fatigue
- Dizziness, pre-syncope, or syncope
- Shortness of breath on exertion
- Reduced exercise tolerance
- Palpitations
If intravascular haemolysis predominates, the patient may complain of back pain and dark urine due to haemoglobinuria.
Clinical examination
On examination, there may be non-specific signs of anaemia including:
- Pallor: of the conjunctiva or palmar creases
- Tachycardia & flow murmurs: due to a hyperdynamic circulation
- High-output cardiac failure: occurs rarely in severe anaemia. Cardiac output is high but is insufficient to meet metabolic demands of the body, causing symptoms and signs of biventricular failure.4
More specific signs of haemolysis include:
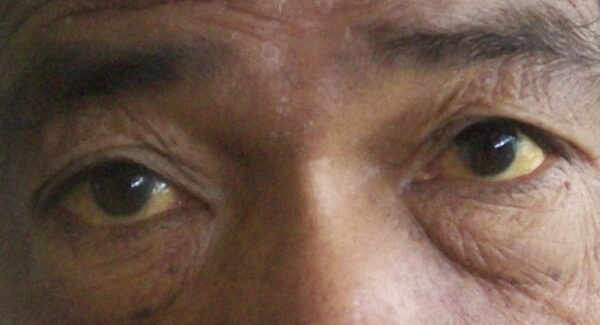
- Pre-hepatic jaundice: due to an unconjugated hyperbilirubinaemia
- Splenomegaly: can be a sign of haemolysis, the underlying cause of haemolysis (especially if massive splenomegaly is present) or can occur due to extramedullary haematopoiesis. This latter phenomenon occurs in chronic, severe haemolytic anaemias such as the haemoglobinopathies when haematopoiesis moves from the bone marrow to the liver and spleen, leading to an increase in their size.
- Dark urine: may be seen in severe haemolysis due to haemoglobinuria, which is most common with intravascular haemolysis
- Gallstones: pigmented gallstones may occur leading to right upper quadrant pain and the other typical signs and symptoms of gallstone disease.
Investigations
To diagnose haemolytic anaemia and its underlying cause, a stepwise approach is taken.
Bedside investigations
Relevant bedside investigations include:
- Urinalysis: in haemolysis there will be increased urinary urobilinogen, but conjugated bilirubin will be negative.
Dipstick testing is highly sensitive for haemolysis, but not very specific, since other conditions may cause a raised urinary urobilinogen including acute hepatitis and cirrhosis.5
The presence of blood on a dipstick urine sample but the absence of red cells on microscopy suggests haemoglobinuria, which is seen in intravascular haemolysis.
Laboratory investigations
Relevant laboratory investigations to confirm red cell breakdown include:1
- Full blood count: there will be anaemia with a normal or increased mean corpuscular volume (MCV). Increased MCV occurs if there is significant reticulocytosis, since reticulocytes are larger cells, and their presence interferes with the MCV measurement of mature erythrocytes.
- Reticulocyte count: the marrow may compensate for increased red cell turnover by increasing haematopoiesis and releasing immature reticulocytes into the peripheral circulation. As such, there may be reticulocytosis.
- Bilirubin: there may be an increased unconjugated (indirect) bilirubin level, occurring due to haemoglobin breakdown.
- Serum lactate dehydrogenase (LDH): LDH is a non-specific marker of cell turnover and is significantly raised in haemolysis due to release from RBCs.
Other tests can be used to determine if the haemolysis is predominantly intravascular or extravascular.
Laboratory features of intravascular haemolysis include:1
- Decreased plasma haptoglobin: haptoglobin is a protein that ‘mops up’ free circulating haemoglobin so that it can be removed by the liver. It will therefore be reduced in intravascular haemolysis as a large amount of free haemoglobin is present in the circulation.
- Urinary dipstick & microscopy: may show haemoglobinuria, which is the red-brown discolouration of urine and a positive dipstick test for blood in the absence of red blood cells on microscopy (Figure 4).
Haemosiderinuria
After several weeks of intravascular haemolysis, haemosiderinuria may occur. This happens when haptoglobin capacity is depleted and free haemoglobin is filtered by the kidneys, accumulating in the renal tubules as haemosiderin.
This is detected in the urine with Prussian blue staining at least one week after onset, as tubular cells slough off into the urine. Haemosiderinuria indicates the presence of chronic intravascular haemolysis (e.g. haemoglobinopathy).
Other investigations
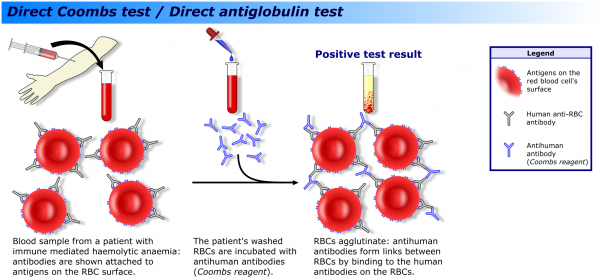
Direct Coombs test
A direct Coombs test (direct antiglobulin test/DAT) is an important part of the haemolysis screen.
The DAT identifies red cells coated with antibody or complement components, which suggests an immune cause for the haemolysis (Figure 5).6
Peripheral blood smear
The peripheral blood smear is another important part of the haemolysis screen. It can give important clues as to the underlying diagnosis. Some features of note include:
- A hypochromic (pale), microcytic (low MCV) picture: can indicate thalassaemia
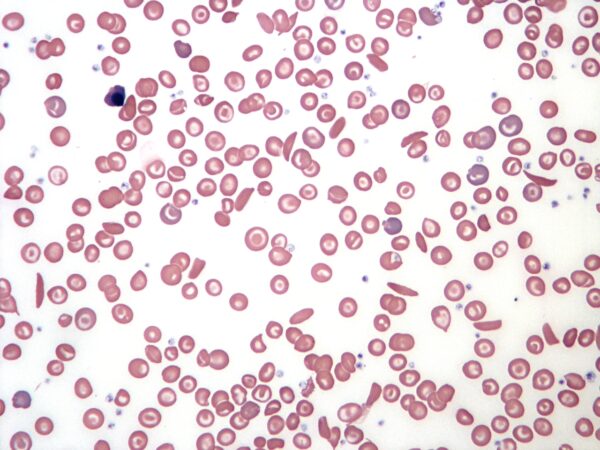
- Sickled red blood cells: pathognomonic of sickle cell anaemia (Figure 6)
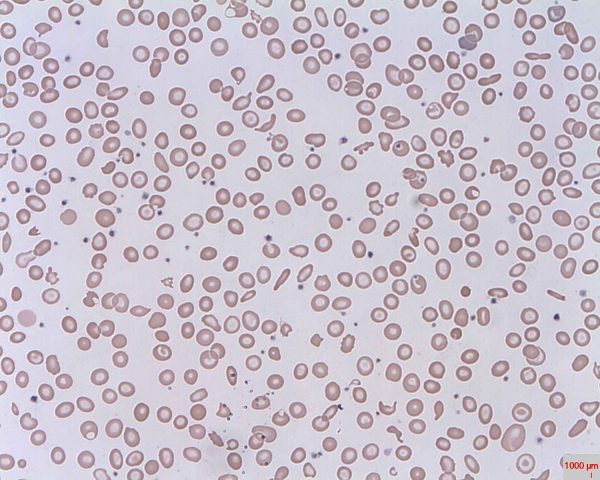
- Schistocytes: fragments of red blood cells that occur when abnormal intravascular shearing forces cause destruction of red cells (Figure 7). These are seen in the microangiopathic haemolytic anaemias (TTP, HUS, HELLP, and DIC).
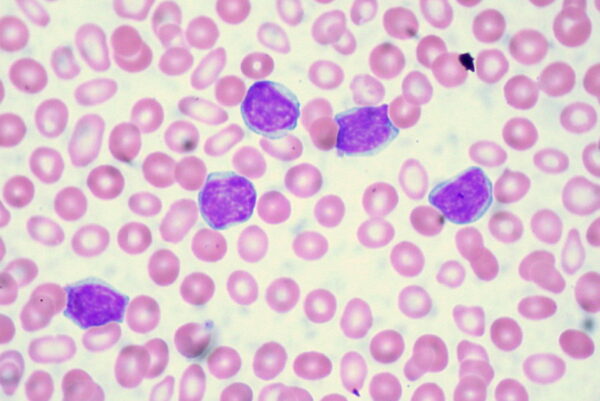
- Malignant cells: haematological malignancy (Figure 8)
- Spherocytes: may be seen in hereditary spherocytosis or in autoimmune haemolysis
- Heinz bodies and ‘bite’ cells: may be seen in G6PD deficiency
Management
The advice of a haematologist should be sought for the management of a patient with haemolytic anaemia. Treatment of the underlying cause should be instigated, which varies depending on the specific diagnosis.
For example, in autoimmune haemolysis steroids with or without intravenous immunoglobulin are the primary treatment. In sickle cell disease, the chemotherapeutic agent hydroxycarbamide reduces haemolysis and crises.
Supportive treatment should also be given including red cell transfusions if the patient has symptomatic anaemia, is actively bleeding or if the haemoglobin concentration is under 70g/L.
Haematinics (B12, iron and folate) should be checked and corrected if low. Folate is also given as standard in chronic haemolysis even if levels are normal to avoid depletion of folate levels due to increased erythropoiesis.
Exchange transfusion can be considered in specific situations. For instance, with life-threatening haemolysis in G6PD deficiency, or in severe sickle cell crises. Advice from a consultant haematologist should be sought in these cases.
Complications
Severe haemolysis can lead to decompensated anaemia with shortness of breath and fatigue. Red cell transfusions are required in these cases.
High-output cardiac failure rarely occurs in particularly severe cases, giving the typical signs and symptoms of congestive cardiac failure. Unlike in other forms of heart failure, cardiac output is preserved. Management involves correcting the anaemia to restore oxygen delivery to the tissues thus reducing myocardial workload.
Pruritus may occur due to jaundice secondary to persistent haemolysis. The cause of haemolysis should be identified and treated if possible. Symptomatic management includes the use of ursodeoxycholic acid and/or cholestyramine.
Pigmented gallstones can occur with chronic haemolysis. Treatment for symptomatic gallstones is with laparoscopic cholecystectomy.
Haemolysis increases the risk of venous thromboembolism. The risk is particularly high in the haemoglobinopathies and in a rare disorder known as paroxysmal nocturnal haemoglobinuria (PNH).
Key points
- Haemolytic anaemia involves a fall in haemoglobin concentration caused by a shortened lifespan of circulating erythrocytes.
- Haemolysis can occur in the vasculature (intravascular) or within the spleen (extravascular), although the latter is more common. Causes can be divided into immune and non-immune conditions.
- Immune conditions include autoimmune haemolysis and alloimmunisation due to transfusion mismatch or haemolytic disease of the newborn.
- Non-immune conditions leading to haemolysis are varied but include haemoglobinopathies, red cell membrane disorders, microangiopathic haemolytic anaemias and red cell enzyme deficiencies.
- Symptoms and signs are those of any anaemia. Dark urine may occur in some patients, particularly if there is intravascular haemolysis.
- Investigations first aim to detect if there is increased red cell turnover (low haemoglobin, raised LDH, reticulocytosis, unconjugated hyperbilirubinaemia).
- Laboratory tests can also determine if haemolysis is predominantly intravascular (low haptoglobins, haemoglobinuria and haemosiderinuria) or extravascular.
- A peripheral blood film and direct antiglobulin test (DAT) are useful tests to ascertain the underlying cause of haemolysis and are therefore routine tests in all patients with suspected haemolysis.
- Treatment depends on the underlying cause. Blood product support may be required in cases of severe symptomatic anaemia and should be guided by local protocols and advice from a haematologist.
- Complications include symptomatic anaemia, high-output cardiac failure, symptomatic jaundice, pigmented gallstones and venous thromboembolism.
Reviewer
Dr James Wilson
Haematology Registrar
Calderdale and Huddersfield NHS Trust
Editor
Dr Chris Jefferies
References
- Wilkinson IB et al., Oxford Handbook of Clinical Medicine. Chapter 8 (Haematology). An Approach to Haemolytic Anaemia. Published in 2017.
- Zivot A et al., Erythropoiesis insights into pathophysiology and treatments in 2017. Molecular Medicine. Published in 2018. Available from: [LINK]
- Nickson C. Haemolytic Anaemia. Life in the Fast Lane (LITFL.com). Published in 2020. Available from: [LINK]
- Singh S et al., High-Output Cardiac Failure. StatPearls. Published in 2021. Available from: [LINK]
- Cadogan M. Dipstick Urinalysis. Life in the Fast Lane (LITFL.com). Published in 2021. Available from: [LINK]
- Samuel R et al., Coombs Test. StatPearls. Published in 2020. Available from: [LINK]
Image references
- Figure 1. Zivot A et al., Overview of Erythropoiesis. License [CC-BY] Available from: [LINK]
- Figure 2. Manning J. Causes of Haemolytic Anaemia.
- Figure 3. Bobjgalindo. Jaundice of the Sclera. License [CC BY-SA]. Available from: [LINK]
- Figure 4. Heilman J. Urine Sample From a Patient with Rhabdomyolysis. License [CC BY-SA]. Available from: [LINK]
- Figure 5. A Rad. Coombs Test Schematic. License [CC BY-SA]. Available from: [LINK]
- Figure 6. Chambers K. Sickle Cell Blood Smear. License [CC BY-SA]. Available from: [LINK]
- Figure 7. Erharbor O. Schistocytes. License [CC BY-SA]. Available from: [LINK]
- Figure 8. Uthman E. Chronic Lymphocytic Leukemia. License [CC BY-SA]. Available from: [LINK]