- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
In 1954, the first kidney transplant was performed by Joseph Murray, between identical twins Ronald and Richard Herrick.1 Almost 70 years later, the kidney is the most transplanted organ across the world with 92,532 transplants taking place in 2021.2
It is the only definitive treatment for end-stage renal failure and can be transformational in terms of quality of life; eliminating the need for dialysis, freeing the recipient from fluid and dietary restrictions and improving life expectancy.
Remarkably, the most common outcome after a kidney transplant is death with a functioning graft.3 In other words, the kidney usually outlives the patient.
Glossary of terms
There are some key terms to understand when discussing transplantation:1
- Allograft: organ or tissue transplanted from a genetically non-identical member of the same species; most kidney transplants are allografts
- Isograft: organ or tissue transplanted from a genetically identical member of the same species (identical twin)
- Xenograft: organ or tissue transplantation from a different species; it is not currently used in clinical practice but played an important part in the development of transplant surgery, and many believe it will play a key role in the future
- Rejection: response of the immune system against the transplanted organ
- Graft: the transplanted organ or tissue
- Warm ischaemia time: the period the donated organ is not perfused but remains at body temperature
- Cold ischaemia time: the period that the donated organ is not perfused and kept cold
- Antibody: protein produced by the immune system to attack specific tissues identified as ‘foreign’
- Antigen: protein which stimulates the immune system to produce antibodies (antibody generating)
- MHC (major histocompatibility complex): a group of genes that code for proteins found on all cells that help the immune system distinguish between self and non-self
- HLA (human leukocyte antigen): the name for the proteins coded for by the MHC in humans
Indications
As renal transplant offers better life expectancy and quality of life than dialysis, all patients with end stage renal failure should be considered for transplantation unless there are absolute contraindications.4
Ideally, the assessment for transplantation should occur pre-emptively before the patient requires renal replacement therapy.
Therefore, all patients with chronic kidney disease stage 4 or 5 that is progressing to the point where they are likely to need renal replacement therapy within six months, should be assessed for transplantation.5
Contraindications
Absolute contraindications
The poorer outcomes of long-term dialysis mean there are few absolute contraindications to renal transplant.
Absolute contraindications include:6
- Untreated malignancy
- Active infection
- Untreated HIV/AIDS
- Life expectancy <2 years
Relative contraindications
Relative contraindications are generally related to whether the patient can tolerate surgery.
Relative contraindications include:6
- Age (>65)
- Untreated coronary artery disease
- Significant obesity
It is also recommended that there be at least two years between remission and transplantation for those with a previous diagnosis of malignancy.6
Finally, patients who have suffered rejection of a previous graft due to poor compliance with immunosuppressive medication need a thorough psychological evaluation before re-listing for transplantation.
Principles of transplantation
The biggest barrier to successful organ transplantation is the recipient’s immune system identifying a transplanted organ as ‘foreign’ and initiating an immune response, a process known as rejection.
The main proteins the immune system uses to determine whether a response is needed are Human Leukocyte Antigens (HLAs).
Two main methods are used to reduce the risk of rejection in renal transplant:
- Reducing the immunogenicity of the graft by assessing and matching donor and recipient HLA types before the operation.
- Lifelong immunosuppressive medications after the operation to prevent an immune response.
Types of donors
As it is perfectly possible to live a healthy life with one functioning kidney, renal transplantation allows for living donors. They account for 30% of all kidney transplants in the UK, and the graft is retrieved on the same day as the transplant operation via a laparoscopic donor nephrectomy.4
The remaining 70% of grafts are provided by deceased donors and are removed by specialist surgeons as part of the organ retrieval team.4
Living donors
Living donors are the gold standard for kidney transplants. They offer the best outcomes in terms of kidney function and both graft and patient survival.
Living donation also reduces the waiting time for the recipient and allows the operation to be planned.4
Deceased donors
Donation after brainstem death (DBD)
Brainstem death occurs when brain injury has led to an irreversible loss of the capacity for consciousness and respiration, but the use of mechanical ventilation has prevented hypoxic cardiac arrest.7
DBD allows retrieval to occur in a controlled manner, with the organs exposed and dissected whilst the heart is still beating, which limits the warm ischaemic time.
Donation after circulatory death (DCD)
In the UK, donation after circulatory death involves the withdrawal of life-sustaining treatment from a critically ill patient where that treatment is deemed to be of no overall benefit.8
Specialist surgeons then wait for asystole to occur (and for a further five minutes after that to confirm death) before beginning the process of organ retrieval. This leads to an increased warm ischaemic time compared to DBD retrieval, reflected in higher rates of delayed graft function. However, long-term outcomes are generally comparable to DBD grafts.8
Expanded criteria donation (ECD)
Expanded criteria donors include those over 60 or those over 55 with comorbidities. ECD donation aims to increase the donor pool, particularly for elderly recipients.3
In the UK today, most deceased donor kidneys are from ECD or DCD donors in an attempt to keep up with demand.
Assessment of the kidney transplant recipient
Kidney transplant recipients need a thorough work-up to ensure they are healthy enough to undergo the operation and receive a well-matched kidney.
- ABO blood group: as with a blood transfusion, the kidney needs to come from a donor with a compatible blood group.
- HLA typing: the most important HLAs for renal transplant are DR, A and B. HLA typing (also known as tissue typing) is reported as the number of mismatches between these antigens, with higher mismatches associated with poorer graft outcomes.9
- Donor-specific antibodies: antibodies against the specific HLA type of the donor. These antibodies can be formed due to exposure to ‘foreign’ tissue via previous organ transplantation, blood transfusion or pregnancy. This is known as sensitisation, and it can be much harder for highly sensitised individuals to find a suitable donor. The formation of these antibodies is one reason it is essential to try to match HLA types between donors and recipients, particularly for younger patients who are likely to need a second transplant in the future.9
- Infection screen: due to the need for ongoing immunosuppression after transplant, patients are screened for infections including HIV, hepatitis B & C, CMV, EBV and VZV.
- General health: as with any major operation, heart and lung function is assessed to ensure the patient can cope with the surgery.
- Psychological evaluation: transplantation and subsequent management can be psychologically demanding, making evaluation important. There also needs to be confidence that the patient will adhere to their post-transplant follow-up regime and medication.
Organ allocation
In an ideal world, each patient requiring a kidney transplant could provide a well-matched living donor. If this is not possible, kidneys are allocated in two main ways.
Living kidney sharing scheme
This system matches incompatible donor/recipient pairs with another pair to overcome the mismatch, a process known as paired donation when two pairs are involved, and pooled donation if the chain is longer.
Living donor kidney matching runs are performed four times per year, allowing more transplants to be performed with grafts from living donors and their associated favourable outcomes.9
Chains can also be started by altruistic donors who offer to donate a kidney without being part of a pair.
National transplant list
Demand for deceased donor kidneys outstrips supply, with over 5,000 people waiting for a graft in the UK.4
Therefore, a fair and transparent system is required to allocate these organs where they benefit most.
All candidates who cannot find a suitable living donor are placed on the national transplant list, which uses a computer algorithm to match them with deceased donor kidneys as they become available.
The algorithm considers how well-matched the donor and recipient are in terms of ABO/HLA types and age, as well as incorporating the time the patient has spent on the transplant list/dialysis, the location of the hospital and how difficult it would be for this patient to get another kidney (for example, if they have a rare blood type or are sensitised to many potential donors).4
The average waiting time for a deceased donor kidney on the national transplant list is two and a half years.10
Operation
Kidney transplant surgery usually takes between two and four hours. A Rutherford-Morrison incision is made in the recipient’s iliac fossa, usually on the right, and the surgeon dissects down to expose the external iliac vessels.
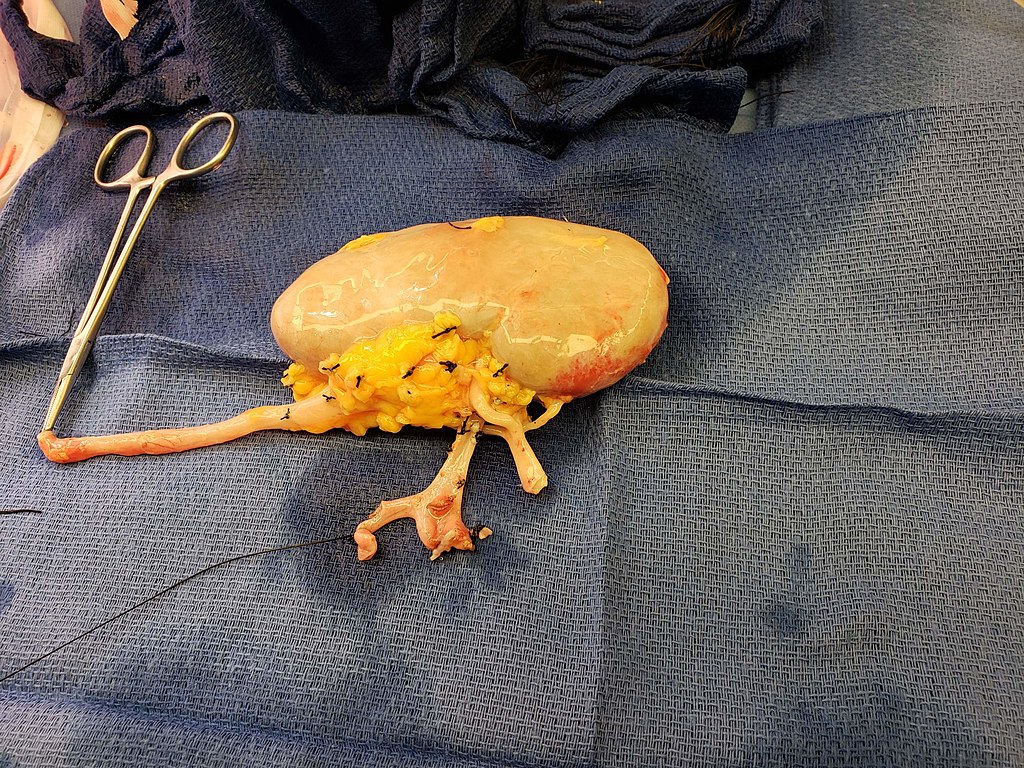
Meanwhile, the donor kidney is prepared on the backbench. Leaks are checked for, and accessory vessels are tied off to ensure graft implantation is as straightforward as possible.
The kidney is placed in the iliac fossa, and three important anastomoses are made; between the renal vessels and their external iliac counterparts and the donor ureter and recipient bladder. A stent is usually placed in this final connection and removed two to six weeks later via cystoscopy.10
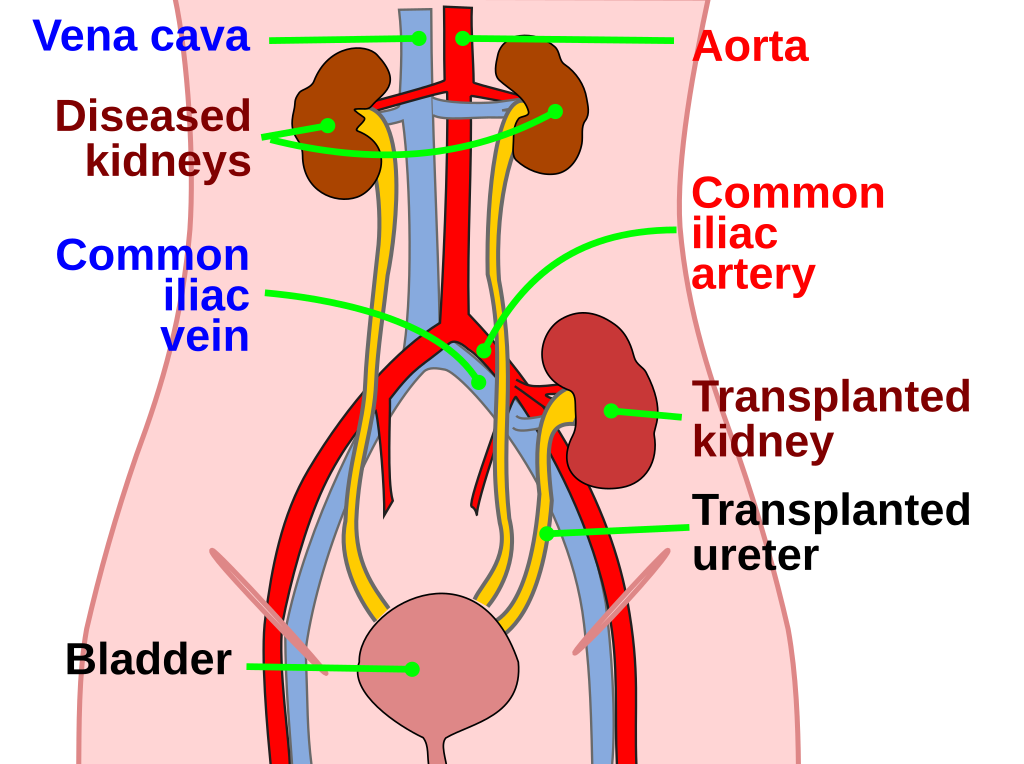
The donor kidney is positioned in the extraperitoneal space, and the native kidneys are usually left in place.11
Post-operative care
After the operation, the patient will have regular serum creatinine measurements to assess renal function and appropriate pain relief.
Polyuria is also common after renal transplant; appropriate IV fluids must be prescribed to prevent pre-renal failure of the graft.12
Most patients can leave the hospital after a week, but twice-weekly follow-up appointments are needed in the first month.
Immunosuppression
All kidney transplant patients (apart from those receiving isograft transplants from an identical twin) will require life-long immunosuppression.3
A combination of medications is given to achieve the required suppression levels with an acceptable side effect profile.
A common combination is triple therapy with a steroid (e.g. prednisolone), a calcineurin inhibitor (e.g. tacrolimus) and an anti-metabolite (e.g. mycophenolate mofetil).
However, the exact combination is tailored to the immune system of the patient, the immunogenicity of the graft and the preferred regime of the individual transplant centre.
After three to six months, the risk of rejection goes down, and doses can usually be reduced.3 The main risks of long-term immunosuppression are malignancy and infection, with additional side effects depending upon the drugs used.
Table 1. Common immunosuppressants used in renal transplantation.
| Class | Example | Mechanism | Side effects |
| Corticosteroid | Prednisolone | Reduces transcription of inflammatory cytokines. | Osteoporosis, weight gain, hypertension, diabetes. |
| Calcineurin inhibitor* | Ciclosporin Tacrolimus |
Inhibits T-cell activation and proliferation | Nephrotoxicity, hypertension and hyperlipidaemia. |
| Anti-metabolite | Mycophenolate mofetil (MMF) Azathioprine |
Inhibits DNA replication, particularly in lymphocytes | Anaemia, leukopenia, gastrointestinal toxicity. |
*Calcineurin inhibitors have a narrow therapeutic index and are metabolised by the CYP450 system. It is important to avoid concurrent prescription drugs which inhibit CYP450 enzymes, such as macrolide antibiotics (e.g. erythromycin).13 Regular tacrolimus ‘trough’ levels are done to ensure correct dosing is achieved and maintained.
Complications
Early complications
Early complications of renal transplantation include:3,4
- Thrombosis: arterial or venous thrombosis can cause rapid graft failure and is identified using renal ultrasound. Patients may need treatment from interventional radiology or further surgery.
- Delayed graft function (DGF): 20-60% of grafts from deceased donors will not function immediately, primarily due to acute tubular necrosis. The risk of DGF is increased by prolonged warm and cold ischaemia times. Fortunately, most grafts will recover and ‘wake up’ within a week, but the patient may need to be placed on haemodialysis.
- Infection: 10-20% of patients may need extra antibiotics in the first week after surgery, most commonly for urinary tract or chest infections
- Bleeding: like any operation, there is a risk of bleeding, and 10% of kidney transplant patients need a blood transfusion in the first week after the operation
- Urine leakage: in 2-3% of patients, there may be urine leakage from the anastomosis between the donor ureter and the recipient bladder. This can be repaired surgically.
- Stenosis: this can occur in any arterial, venous or ureteric anastomoses and may need to be treated with balloon dilatation +/- stent insertion.
Late complications
Late complications of renal transplantation include:3,4,14
- Cancer: particularly cancer associated with viruses due to long-term immunosuppression. For example, transplant patients have a higher risk of skin cancer (HPV), lymphoma (EBV) and Karposi Sarcoma (HHV8). There is also a slight increase in other cancers due to the immune system’s role in cancer surveillance. Patients should be encouraged to attend national screening programmes and avoid sun exposure.
- Infection: increased risk of opportunistic infection due to immunosuppression, particularly CMV (cytomegalovirus) and pneumocystis pneumonia, for which prophylactic medication may be given
- Recurrence of renal disease: the likelihood of disease recurrence depends upon the cause. Primary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis and diabetic nephropathy have a higher risk of recurrence.
- Cardiovascular disease: this is the most common cause of mortality after a renal transplant, accounting for 50% of transplant patient deaths
Rejection
Rejection is a key complication of kidney transplant surgery. Depending upon the mechanism, it can occur at different times during the post-operative period, with the risk of rejection highest in the first three months after the operation.
A percutaneous kidney biopsy is required to confirm a diagnosis of rejection.4
Hyperacute rejection
Occurs minutes to hours after the operation and leads to rapid and irreversible graft failure.
Hyperacute rejection is mediated by pre-existing antibodies against donor HLA antigens or an incompatible donor ABO blood type. It is rarely seen in practice due to screening for sensitisation and a crossmatch test directly before the transplant (mixing donor lymphocytes with recipient serum; a positive crossmatch is a contraindication to transplantation).9
Acute rejection
This is the most common form of graft rejection, usually occurring in the first thirty days after transplant.
It often results in an asymptomatic decline in graft function, so patients are screened regularly, particularly in the first weeks after the operation. Acute rejection is most commonly T-cell mediated and is usually amenable to increased immunosuppression.14
Chronic rejection
Rejection after at least six months with smooth muscle proliferation, interstitial fibrosis and scarring. This process is not fully understood and is likely due to a combination of immunological damage, hypertension and chronic drug toxicity. Unfortunately, there are limited treatment options available.14
Prognosis following kidney transplantation
Currently, 99% of recipients who receive grafts from living donors are alive at one year and 86% at ten years, compared to 97% and 76% for deceased donors.4
Kidneys from living donors also tend to function longer, an average of 20-25 years, compared with 15-20 years for kidneys from deceased donors.4
However, improved surgical techniques and immunosuppression, along with greater compatibility between donor and recipient as a result of more appropriate patient selection and assessment, mean that outcomes for renal transplant from both living and deceased donors continue to improve.3
Key points
- Renal transplant is the only definitive treatment for end-stage renal failure
- All patients progressing to a point where they are likely to need renal replacement therapy within six months should be assessed for transplantation
- There are few absolute contraindications to renal transplantation
- Kidney donors can be living or deceased, with the best outcomes associated with living donors
- Rejection is a key complication of renal transplant and can be hyperacute, acute or chronic, depending on the mechanism
- The risk of rejection can be reduced by ensuring the graft is well-matched to the recipient alongside lifelong immunosuppression
- The key risks of long-term immunosuppression are malignancy and infection
- The most common cause of death in patients with renal transplants is cardiovascular disease
- The most common outcome after a renal transplant is death with a functioning graft
Reviewer
Miss Emily Thompson
NIHR Clinical Lecturer in Transplant Surgery
Editor
Dr Chris Jefferies
References
- Mezrich J. How Death Becomes Life: Notes from a Transplant Surgeon. London: Atlantic Books; 2019. 384 p.
- Global Observatory on Donation and Transplantation. Kidney Transplants. Published in 2021. Available from: [LINK]
- Yaqoob M. Magdi & Ashmen N. Kidney and Urinary Tract Disease. In: Feather A, Randall D, Waterhouse M, editors. Kumar and Clark’s Clinical Medicine. 10th London: Elsevier; 2021. p.1339-1408.
- NHS Blood and Transplant. Kidney Transplant. Available from: [LINK]
- Thiruchelvam PTR, Willicombe M, Hakim N, Taube D, Papalois V. Renal Transplantation. BMJ. 2011 Nov 19. 343:1055-1059.
- NHS Blood and Transplant. Patient Selection for Deceased Donor Kidney Only Transplantation. Available from: [LINK]
- NHS Blood and Transplant. Donation after Brainstem Death. Published in 2023. Available from: [LINK]
- NHS Blood and Transplant. Donation after Circulatory Death. Published in 2023. Available from: [LINK]
- Kidney Care UK. Kidney Transplants: What you need to know about blood groups, antibodies and sensitization. Published in 2023. Available from: [LINK]
- NHS.uk. Kidney Transplant. Published in 2022. Available from: [LINK]
- Conway B, Phelan PJ & Stewart GD. Nephrology and Urology. In: Penman ID, Ralston SH, Strachan MWJ, Hobson RP, editors. Davidson’s Principles and Practice of Medicine. 24th London: Elsevier; 2023. p. 557-611.
- Agarwal A, Jeyarajah S, Harries R, Weerakkody R, McLatchie G & Borley N. Oxford Handbook of Clinical Surgery. 5th Oxford: OUP; 2022. 1056 p.
- Wilkinson IB, Raine T, Wiles K, Goodhart A, Hall C & O’Neill H. Oxford Handbook of Clinical Medicine. 10th Oxford: OUP; 2017. 894 p.
- Johnston SL. Clinical Immunology. In: Penman ID, Ralston SH, Strachan MWJ, Hobson RP, editors. Davidson’s Principles and Practice of Medicine. 24th London: Elsevier; 2023. p.59-86.
Image references
- Figure 1. Wiremu Stadtwald Demchick. Kidney location after transplantation. License: [CC BY]
- Figure 2. Rmarlin. Kidney for transplant from live donor. License: [CC BY-SA]