- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
The urea & electrolytes (U&E) profile is one of the most common blood test investigations, often called simply ‘U&Es’. A standard urea and electrolytes profile includes:
- Serum creatinine
- Estimated glomerular filtration rate (eGFR)
- Serum urea
- Serum sodium
- Serum potassium
This guide gives an overview of the U&E panel and provides a structured approach to U&E interpretation.
Why check U&Es?
U&Es are commonly ordered to assess renal (kidney) function. The kidney excretes urea and creatinine, and these can be used as surrogate markers of renal function. Sodium and potassium are included in the panel, as renal dysfunction can lead to electrolyte derangements.
Other reasons to order U&Es include suspected electrolyte disturbances due to non-renal causes, medication monitoring (e.g. after starting ACE inhibitors), or assessing urea levels in the case of suspected upper gastrointestinal haemorrhage.
Drug monitoring
Common drugs which require monitoring of U&Es include:
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)
- Diuretics: spironolactone, thiazide diuretics (e.g. indapamide), loop diuretics (e.g. furosemide)
- Direct-acting oral anticoagulants (DOACs)
- Carbamazepine
- Lithium
- Digoxin
Reference ranges
Table 1. Urea & electrolytes profile reference ranges
| Test | Reference range |
| Creatinine |
|
| eGFR | >90ml/min/1.73m3 |
| Urea | 2.5 – 7.8 mmol/L |
| Sodium | 135–146 mmol/L |
| Potassium | 3.5–5.3 mmol/L |
These reference ranges can vary between laboratories, so always check local guidelines.
Creatinine
Creatinine is a waste product of muscle metabolism excreted entirely by the kidney.1
Serum creatinine level is naturally higher in individuals with greater skeletal muscle mass (thus generally higher in males than females). However, the primary determinant of serum creatinine is the kidney’s ability to filter creatinine from the bloodstream.
Therefore, a raised creatinine level is an indicator of kidney dysfunction.
Estimated Glomerular Filtration Rate (eGFR)
The eGFR is a mathematically derived number based on a patient’s serum creatinine in conjunction with age, sex and race.2
eGFR aims to estimate the glomerular filtration rate (GFR), which cannot be directly measured in humans. As the serum creatinine rises, the eGFR will decrease, indicating worsening kidney dysfunction.
A ‘normal’ glomerular filtration rate is around 100ml/min/1.73m3. When discussing results with patients, the eGFR can be roughly equated to a percentage of kidney function.
This is particularly relevant in chronic kidney disease (CKD), where the eGFR is used to ‘stage’ the disease:
- Stage 1: eGFR >90 (normal), with other tests showing signs of kidney damage (e.g. proteinuria)
- Stage 2: eGFR of 60 to 89 ml/min, with other tests showing signs of kidney damage (e.g. proteinuria)
- Stage 3a: eGFR of 45 to 59 ml/min
- Stage 3b: eGFR of 30 to 44 ml/min
- Stage 4: eGFR of 15 to 29 ml/min
- Stage 5: eGFR <15 ml/min
Problems with eGFR
The most common equations used to calculate eGFR are the MDRD and CKD-EPI equations. The CKD-EPI differs from the MDRD equation because it does not add a race factor to the calculation.
Both equations are well-validated. However, in some circumstances, the eGFR can vary significantly from the true glomerular filtration rate (e.g. patients with extremes of body type – malnourished, bodybuilders, severe obesity or limb amputees). The equations are also not valid for those under 18 years of age.
Urea
Urea is a waste product of protein breakdown produced in the liver. 3 The kidneys predominantly excrete urea, and it can be used as a surrogate marker of renal function. However, this is fairly non-specific.
Causes of a raised serum urea (uraemia) include:
- Renal dysfunction: decreased excretion of urea into the urine.
- Dehydration: urea rises quickly in dehydration, even in the presence of normally functioning kidneys. This is physiologically mediated by anti-diuretic hormone (ADH), released from the posterior pituitary gland in response to intravascular volume depletion. ADH increases urea and water reabsorption in the collecting ducts.
- Upper gastrointestinal bleeding: blood in the upper GI tract is broken down into proteins, which are transported to the liver via the portal vein and metabolised into urea.
Low serum urea levels are non-pathological, associated with pregnancy and those on a low-protein diet.
Sodium
Sodium is the main determinant of plasma osmolality, and serum levels are closely related to hydration (volume) status. Serum sodium levels are normally regulated by antidiuretic hormone (ADH), amongst other homeostatic mechanisms.
Symptoms of both hypernatremia and hyponatremia are primarily neurological. Mild symptoms include fatigue, weakness and confusion, but can progress to severe symptoms such as seizures and coma.4
The severity of symptoms is related to the severity of sodium derangement and the pace of the change in serum sodium concentration (rapid changes lead to severe symptoms).
Hypernatraemia
Hypernatraemia is defined as a serum sodium level >146mmol/L. It is most commonly caused by dehydration (e.g. unreplaced skin or GI losses of hypotonic fluid).5
Rarer causes of hypernatraemia include:
- Diabetes insipidus
- Drugs (e.g. loop diuretics)
- Osmotic diuresis (e.g. in hyperglycemic states)
- Extreme levels of salt ingestion
In most cases, where there is a clear history implicating dehydration (e.g. vomiting/diarrhoea), treatment involves gentle rehydration with intravenous hypotonic fluids (e.g. 5% dextrose).
It is essential to monitor sodium levels during treatment, as correcting the hypernatraemia too rapidly can lead to intracerebral fluid shifts and devastating consequences such as central pontine myelinolysis (also known as osmotic demyelination syndrome).
Hyponatraemia
Hyponatraemia is defined as a serum sodium level <135mmol/L. It is often caused by a failure to excrete water normally (e.g. SIADH).6
Treatment can vary according to the underlying cause and the patient’s fluid status. Again, it is important to regularly monitor sodium levels to avoid rapid correction.
For more information, see our guide to hyponatraemia.
Potassium
Derangements in potassium levels are common in hospital inpatients. Under physiological conditions, potassium is stored almost entirely intracellularly and is excreted by the kidneys. Derangements in potassium levels cause myocardial instability, leading to risks of potentially fatal arrhythmias.
Hyperkalaemia
Hyperkalaemia is defined as serum potassium >5.5mmol/L. It can be further classified in terms of severity as per European Resuscitation Council guidelines:
- Mild: 5.5-5.9 mmol/L
- Moderate: 6.0-6.4 mmol/L
- Severe: >6.5 mmol/L
Causes of hyperkalaemia can be varied:
- Renal: decreased renal excretion (e.g. AKI or CKD)
- Medications: ACE inhibitors, potassium-sparing diuretics, potassium supplements
- Tissue damage: burns, rhabdomyolysis
- Metabolic: diabetic ketoacidosis
- Endocrine: Addison’s disease
Severe hyperkalaemia is a medical emergency requiring immediate management. If ECG changes occur, the most critical initial step is administering intravenous calcium gluconate, stabilising the myocardium. Once this is administered, treatment focuses on lowering the serum potassium concentration, often via insulin/dextrose infusion.
For more information, see our guide to hyperkalaemia.
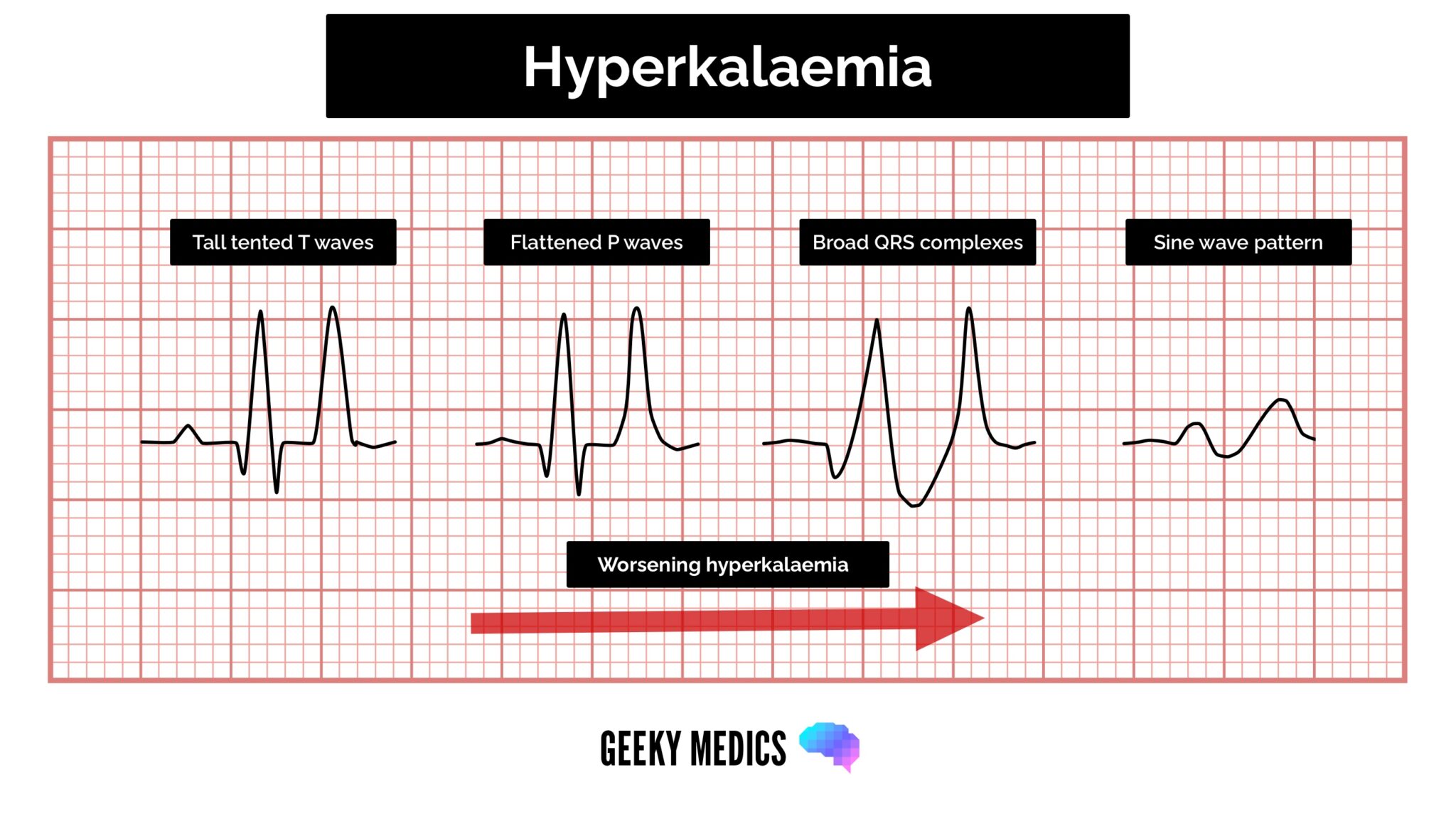
ECG features of hyperkalaemia
The cardinal features of hyperkalaemia on the ECG are peaked T waves, prolonged PR interval and widened QRS complexes.
Hypokalaemia
Hypokalaemia is defined as serum potassium <3.5mmol/L. Severe hypokalaemia is defined as a serum potassium level <3.0mmol/L, which can destabilise the myocardium.
ECG changes associated with hypokalaemia include PR prolongation, widespread ST depression / T wave flattening and prominent U waves.
Causes of hypokalaemia
Gastrointestinal potassium loss
Lower gastrointestinal losses are relatively high in potassium, and prolonged diarrhoea can cause significant hypokalaemia.
Upper gastrointestinal losses are relatively low in potassium. However, prolonged vomiting can also lead to hypokalaemia. This occurs due to metabolic alkalosis secondary to gastric acid loss. Excess potassium is excreted from the kidney as part of the renal compensatory mechanism to correct the alkalosis.
Renal potassium loss
Renal potassium wasting occurs most commonly due to the effect of medications such as loop diuretics (e.g. furosemide) and thiazide diuretics (e.g. indapamide). Other causes include increased mineralocorticoid activity or renal tubular acidosis (type I / II).
Intracellular potassium accumulation
High extracellular pH (alkalosis), elevated beta-adrenergic activity or increased availability of insulin will act to drive potassium into cells.
Management
Management of hypokalaemia involves intravenous or oral potassium replacement alongside treatment of the underlying cause:8
- If potassium is >3mmol/L, the patient is asymptomatic, and there are no ECG changes, oral potassium replacement can be given.
- If potassium is <3mmol/L (severe hypokalaemia) or ECG changes are present, intravenous potassium replacement is indicated.
Approach to U&E interpretation
Step 1: Assess urea, creatinine & eGFR
Assessing the urea and creatinine in conjunction can allow you to determine whether there is potential kidney injury/damage. If both are raised, this suggests renal dysfunction and the eGFR will be low.
Remember, an isolated raised urea can indicate dehydration or upper gastrointestinal bleeding.
Step 2: Compare to previous creatinine / eGFR
Without a factor leading to an inaccurate eGFR calculation (e.g. obesity), a high creatinine and low eGFR suggest renal dysfunction.
It is then important to assess whether this is acute (i.e. acute kidney injury) or longstanding (i.e. chronic kidney disease).
AKI vs CKD
Acute kidney injury (AKI) is a rapid worsening of renal function and a common complication among hospital inpatients. It is most commonly diagnosed when there is a creatinine rise of 1.5x from baseline and can be staged according to the severity of the creatinine rise.
Chronic kidney disease (CKD) can be diagnosed when two blood tests, three months apart, show a reduction in renal function, leading to health implications. Again, the severity of creatinine rise (and thus eGFR fall) is used to stage the disease.
Step 3: Assess electrolytes
Both AKI and CKD can be associated with electrolyte disturbances, which can be life-threatening (e.g. severe hyperkalaemia). Once you have established a diagnosis of AKI or CKD, assessing sodium and potassium levels and appropriately managing abnormalities is important.
Tip: In renal dysfunction, there can also be significant disturbances in calcium and phosphate levels, therefore it is important to request a bone profile.
Editor
Dr Chris Jefferies
References
- UpToDate. Assessment of kidney function. March 2023. Available from: [LINK]
- UK Kidney Association. About eGFR. June 2023. Available from: [LINK]
- South Tees Hospitals NHS Foundation Trust. Urea. April 2022. Available from: [LINK]
- UpToDate. Manifestations of hyponatremia and hypernatremia in adults. January 2022. Available from: [LINK]
- UpToDate. Treatment of hypernatremia in adults. Sept 2021. Available from: [LINK]
- UpToDate. Diagnostic evaluation of adults with hyponatremia. June 2022. Available from: [LINK]
- UpToDate. Causes of hypokalemia in adults. May 2022. Available from: [LINK]
- UpToDate. Clinical manifestations and treatment of hypokalemia in adults. November 2021. Available from: [LINK]