- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Chronic heart failure (CHF) is a clinical syndrome involving reduced cardiac output because of impaired cardiac contraction. Typical clinical symptoms of CHF include shortness of breath, fatigue and ankle swelling.1
CHF prevalence is 1-2%, rising to 10% in over 70-year-olds.4
For more information on the acute presentation of heart failure, see the Geeky Medics guide to acute heart failure.
Aetiology
Pathophysiology
Stroke volume requires:
- adequate preload
- optimal myocardial contractility (Frank-Starling mechanism)
- decreased afterload
As a result, cardiac output (CO) can be reduced by any of the following factors (potentially causing CHF):
- decreased heart rate
- decreased preload
- decreased contractility
- increased afterload
Cardiac output (CO) = Heart rate (HR) x Stroke volume (SV)
Causes of heart failure
The most common causes of heart failure in the UK are coronary heart disease (myocardial infarction), atrial fibrillation, valvular heart disease and hypertension.
Other causes of heart failure include:
- Endocrine disease: hypothyroidism, hyperthyroidism, diabetes, adrenal insufficiency, Cushing’s syndrome
- Medications: calcium antagonists, anti-arrhythmics, cytotoxic medication, beta-blockers.
High-output cardiac failure occurs in states where demand exceeds normal cardiac output such as pregnancy, anaemia and sepsis.
HIGH-VIS
The acronym HIGH-VIS is useful to remember some of the causes of CHF:
- Hypertension (common cause)
- Infection/immune: viral (e.g. HIV), bacterial (e.g. sepsis), autoimmune (e.g. lupus, rheumatoid arthritis)
- Genetic: hypertrophic obstructive cardiomyopathy (HOCM), dilated cardiomyopathy (DCM)
- Heart attack: ischaemic heart disease (common cause)
- Volume overload: renal failure, nephrotic syndrome, hepatic failure
- Infiltration: sarcoidosis, amyloidosis, haemochromatosis
- Structural: valvular heart disease, septal defects
Clinical features
History
Patients with CHF often present with symptoms that have gradually worsened over months to years.
Typical symptoms of CHF include:
- Dyspnoea on exertion
- Fatigue limiting exercise tolerance
- Orthopnoea: the patient may be using several pillows to reduce this symptom.
- Paroxysmal nocturnal dyspnoea (PND): attacks of severe shortness of breath in the night that are relieved by sitting up (pathognomonic for CHF).
- Nocturnal cough with or without the characteristic ‘pink frothy sputum’.
- Pre-syncope/syncope
- Reduced appetite
Other important areas to cover in the history include:
- Past medical history: hypertension, coronary artery disease and valvular heart disease (common causes of CHF)
- Medication history: several medications can cause or worsen CHF including calcium antagonists, antiarrhythmics, cytotoxic medication and beta-blockers (in the acute phase, but long term provide prognostic benefit).
- Family history: specifically close relatives with cardiac issues such as cardiomyopathy (e.g. HOCM) or coronary artery disease.
- Social history: risk factors for CHF include smoking, excess alcohol intake and recreational drug use.
Clinical examination
Clinical findings on cardiovascular examination may include:
- Tachycardia at rest
- Hypotension
- Narrow pulse pressure
- Raised jugular venous pressure
- Displaced apex beat (due to left ventricular dilatation)
- Right ventricular heave
- Gallop rhythm on auscultation (pathognomic for CHF)
- Murmurs associated with valvular heart disease (e.g. an ejection systolic murmur in aortic stenosis)
- Pedal and ankle oedema
Clinical findings on respiratory examination may include:
- Tachypnoea
- Bibasal end-inspiratory crackles and wheeze on auscultation of the lung fields
- Reduced air entry on auscultation with stony dullness on percussion (pleural effusion)
Clinical findings on abdominal examination may include:
- Hepatomegaly
- Ascites
Investigations
After a comprehensive history and clinical examination have been performed, the following investigations are recommended by NICE.2
Bedside investigations
Relevant bedside investigations include:
- ECG: should be performed on all patients with suspected heart failure. An ECG may identify evidence of previous myocardial infarction (e.g. ‘Q’ waves) or arrhythmias (AV block or atrial fibrillation). A normal ECG makes heart failure unlikely.1
- Urinalysis: may show glycosuria (diabetes) or proteinuria (renal disease)
ECG findings
ECG findings associated with heart failure include:
- Tachycardia
- Atrial fibrillation (due to enlarged atria)
- Left-axis deviation (due to left ventricular hypertrophy)
- P wave abnormalities (e.g. P.mitrale/P.pulmonale due to atrial enlargement)
- Prolonged PR interval (due to AV block)
- Wide QRS complexes (due to ventricular dyssynchrony)
Laboratory investigations
Relevant laboratory investigations include:
- FBC: anaemia
- U&Es: renal failure, electrolyte abnormalities due to fluid overload (e.g. hyponatraemia)
- LFTs: hepatic congestion
- Troponin: if considering recent myocardial infarction
- Lipids/HbA1c: ischaemic risk profile
- TFTs: hyperthyroidism/hypothyroidism
- Cardiomyopathy screen (see below)
- N-terminal pro-B-type natriuretic peptide (see below)
Cardiomyopathy screen
Screening for cardiomyopathy includes the following blood tests:
- Serum iron and copper studies (to rule out haemochromatosis and Wilson’s disease)
- Rheumatoid factor, ANCA/ANA, ENA, dsDNA (to rule out autoimmune disease)
- Serum ACE (to rule out sarcoidosis)
- Serum-free light chains (to rule out amyloidosis)
NT-proBNP
N-terminal pro-B-type natriuretic peptide (NT-proBNP) should be measured in all patients presenting with symptoms and clinical signs of heart failure to inform the type and urgency of further investigations such as echocardiography:
- NT-proBNP level >2000 ng/L – refer urgently for specialist assessment and transthoracic echocardiography within 2 weeks
- NT-proBNP level 400-2000ng/L – refer routinely for specialist assessment and transthoracic echocardiography within 6 weeks
- NT-proBNP level <400 ng/L – heart failure unlikely
Other conditions in which NT-proBNP may be raised include:
- Left ventricular hypertrophy
- Tachycardia
- Liver cirrhosis
- Diabetes
- Acute or chronic renal disease
Imaging
Echocardiography
All patients with suspected chronic heart failure should undergo transthoracic echocardiography, with the urgency determined by their NT-proBNP level as discussed above.
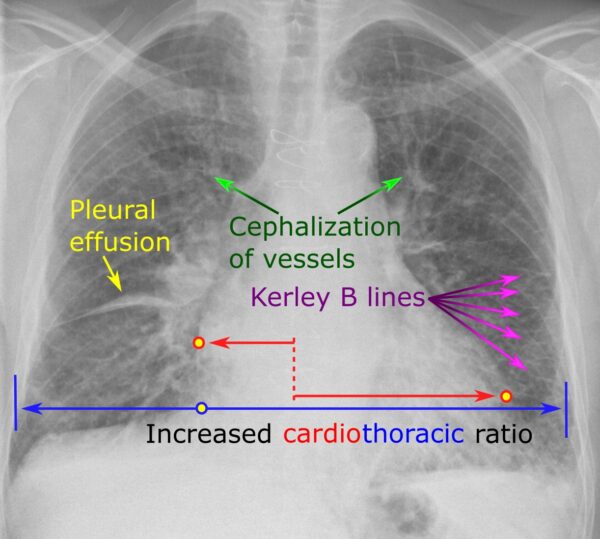
Chest X-ray
Typical chest X-ray findings associated with CHF include:
- Alveolar oedema (perihilar/bat-wing opacification)
- Kerley B lines (interstitial oedema)
- Cardiomegaly (cardiothoracic ratio >50%)
- Dilated upper lobe vessels
- Effusions (e.g. pleural effusions – blunted costophrenic angles)
Cardiac MRI
Cardiac MRI is the gold standard investigation for assessing ventricular mass, volume and wall motion. It can also be used with contrast to identify infiltration (e.g. amyloidosis), inflammation (e.g. myocarditis) or scarring (e.g. myocardial infarction). It is typically used when echocardiography has provided inadequate views.5
Classification
Structural classification
Chronic heart failure can be classified structurally based on left ventricular ejection fraction (LVEF).
LVEF is the percentage of blood that enters the left ventricle in diastole that is subsequently pumped out in systole.
LVEF is usually measured using transthoracic echocardiography, however, MRI, nuclear medicine scans and transoesophageal echocardiography can also be used.1,2
Symptomatic/functional classification
The New York Heart Association’s (NYHA) classification system relies on patient symptoms and level of function:3
- Class I: no symptoms during ordinary physical activity
- Class II: slight limitation of physical activity by symptoms
- Class III: less than ordinary activity leads to symptoms
- Class IV: inability to carry out any activity without symptoms
Management
The focus of CHF management is to improve cardiac function and quality of life, prevent hospitalisation and reduce mortality.
General management
Lifestyle management
Lifestyle management strategies include:
- Fluid and salt restriction
- Regular exercise
- Smoking cessation
- Reduced alcohol intake
Vaccination
All patients with CHF should be offered vaccination for influenza and pneumococcal disease.
Medication review
A medication review should be performed to identify medications which may be harmful in the context of heart failure such as:
- Calcium channel blockers (e.g. verapamil, diltiazem)
- Tricyclic antidepressants
- Lithium
- NSAIDs and COX-2 inhibitors
- Corticosteroids
- QT-prolonging medications
Monitoring
All patients with chronic heart failure require monitoring of:
- Functional capacity, fluid status, cardiac rhythm, cognitive status and nutritional status
- Renal function
The frequency of monitoring depends on the patient’s clinical condition.
Management of co-morbidities
Coronary artery disease
If heart failure is caused by coronary artery disease, statins and aspirin may be prescribed as secondary prevention.
Atrial fibrillation
Oral anticoagulation is recommended for patients with heart failure and atrial fibrillation (either paroxysmal or permanent) due to the high risk of stroke.
Pharmacological management
Pharmacological treatment aims to increase cardiac output by optimising preload and contractility whilst decreasing afterload.
The medications below target the pathological sympathetic response and renin-angiotensin-aldosterone system (RAAS) activation that occurs in CHF.
Diuretics
Diuretics should be prescribed to relieve symptoms of fluid overload (e.g. shortness of breath, peripheral oedema).
Diuretics (e.g. furosemide) work by increasing sodium excretion via diuresis, ultimately reducing cardiac afterload.
Doses should be titrated according to clinical response and renal function should be closely monitored.
ACE inhibitors
All patients with CHF and a reduced ejection fraction (≤40%) should be commenced on an ACE inhibitor unless contraindicated.
ACE inhibitors have been shown to improve ventricular function and reduce mortality.
U&Es should be checked prior to starting treatment and then after 1-2 weeks of treatment.
Contraindications include a history of angioedema, bilateral renal artery stenosis, hyperkalaemia (>5 mmol/L), severe renal impairment (serum creatinine >220 μmol/L) and severe aortic stenosis.
Beta-blockers
Beta-blockers (e.g. bisoprolol) should be prescribed for all patients with symptomatic heart failure and reduced LVEF (≤40%) unless contraindicated.
Beta-blockers decrease heart rate, myocardial oxygen demand and RAAS activation.
Blood pressure and heart rate need to be monitored carefully when adjusting doses.
Contraindications include asthma, 2nd or 3rd degree AV block, sick sinus syndrome and sinus bradycardia.
Angiotensin-II receptor antagonists (ARBs)
If a patient is unable to tolerate an ACE inhibitor (usually due to persistent cough) an ARB (e.g. candesartan) should be prescribed as an alternative.
Patients must have normal serum potassium and adequate renal function to commence an ARB.
Mineralocorticoid/aldosterone receptor antagonists (MRAs)
A low-dose aldosterone antagonist (e.g. spironolactone or eplerenone) should also be prescribed if a patient continues to have symptoms of heart failure despite diuretics, ACE inhibitors and beta-blockers.
MRAs antagonise aldosterone, increasing sodium excretion via diuresis, ultimately decreasing cardiac afterload.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors
SGLT2 inhibitors (e.g. dapagliflozin) can be used as add-on therapy in patients with a reduced LVEF (<40%). Dapagliflozin has been shown to reduce the risk of cardiovascular events and hospital admission. This benefit occurs regardless of the patient’s glycaemic control.5
Specialist pharmacological treatments
Ivabradine
Ivabradine inhibits the sinoatrial node, slowing the heart rate of patients in sinus rhythm, increasing stroke volume whilst preserving myocardial contractility.
It has been shown to reduce cardiovascular death or hospitalisation for heart failure by 18%.
ARNI (angiotensin receptor and neprilysin inhibitor)
ARNI’s increase BNP levels by inhibiting the neprilysin enzyme which breaks down BNP.
Higher BNP causes natriuresis/diuresis, therefore decreasing cardiac afterload.
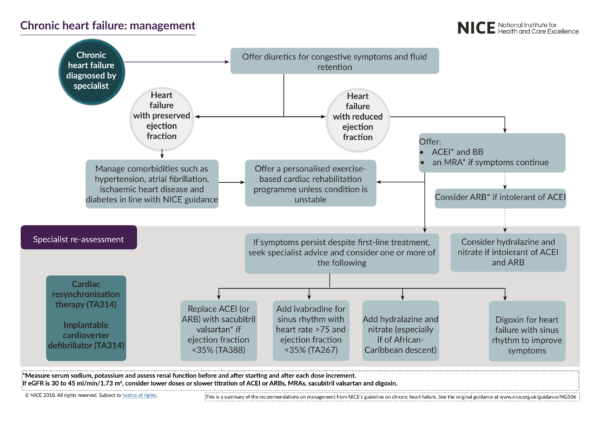
Other management options
If heart failure is caused or worsened by other conditions, these should be managed appropriately:2
- Revascularisation (e.g. coronary artery bypass grafting)
- Valve surgery (e.g. aortic valve replacement)
- Implantable cardiac defibrillator (ICD): inserted if EF <30% for prevention of fatal arrhythmias
- Cardiac resynchronisation therapy + defibrillator (CRT-D): a biventricular pacemaker for EF <30% + QRS >130 m/sec to re-synchronise left and right ventricular contraction to improve EF
- Cardiac transplantation is rare and strict criteria must be met for consideration.
Complications
Complications of CHF include:
- Arrhythmias: atrial fibrillation and ventricular arrhythmias
- Depression and impaired quality of life
- Loss of muscle mass
- Sudden cardiac death
Prognosis is poor overall, with approximately 50% of people with heart failure dying within five years of diagnosis.7
Key points
- Chronic heart failure (CHF) is a clinical syndrome resulting in reduced cardiac output as a result of impaired cardiac contraction.
- The most common causes of heart failure in the UK are coronary heart disease (myocardial infarction, atrial fibrillation, heart block) and hypertension.
- Typical symptoms of CHF include shortness of breath, fatigue and ankle swelling.1
- Investigations required for diagnosis include ECG, NT-proBNP and echocardiography.
- Management involves a combination of lifestyle modification, pharmacological therapies and in some cases surgical intervention.
- The prognosis of CHF is generally poor with sudden cardiac death common.
Reviewer
Dr Steven Sutcliffe
Consultant Cardiologist
Editor
Dr Chris Jefferies
References
- European Society of Cardiology. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Published in 2016. Available from [LINK].
- Chronic heart failure in adults – diagnosis and management; NICE Guidance (Sept 2018). Available from: [LINK]
- Penn Medicine. Heart Failure Classification – Stages of Heart Failure and Their Treatments. Published in 2014.
- Mikael Häggström. Public domain. Available from: [LINK]
- NICE. Dapagliflozin for treating chronic heart failure with reduced ejection fraction. 2021. Available from: [LINK]
- NICE. Visual summary of chronic heart failure management. All rights reserved. Subject to notice of rights.
- Dr Colin Tidy. Patient.info. Heart failure. Published November 2018. Available from: [LINK]