- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
The word “Menopause” comes from the Greek word “Men”, meaning the moon or the months and another Greek word “pauein”, meaning stop.1
Menopause is the final menstrual period, indicating the start of a non-pathological state of permanent amenorrhoea.2 The average age of menopause in the UK is 52.3 It is accompanied by a fall in oestrogen levels, which may result in related symptoms and the need for Hormone Replacement Therapy (HRT). It can only be diagnosed one year after the final menstrual period.
This article will cover the physiology of menopause and the physiology of menopausal symptoms.
Physiology of menopause
Follicular development2,4,5
Where do egg cells come from?
During embryological development, primordial germ cells (undifferentiated stem cells that develop into gametes) in the developing gonads undergo mitosis. These cells are known as oogonia. At 12 weeks gestation, they enter meiosis but stop at prophase 1, becoming primary oocytes. Primary oocytes are surrounded by a single layer of flat granulosa cells. This structure is called a primordial follicle.
The follicles are surrounded by ovarian stroma, which contains interstitial cells, contractile cells, and connective tissue. The former differentiate into theca cells, and the latter provide structural support to the ovaries. The ovarian stroma is vascular, unlike the preovulatory follicles, which are separated from the stroma by a thin basement membrane and rely upon the formation of gap junctions and diffusion to receive nutrients, hormones and experience the effect of those hormones.
A female is born with all the oocytes she will ever have. No new oocytes are made after birth. A female foetus has four million oocytes, at birth this becomes one/two million. Each month before puberty, this number decreases by 10,000. Only around 400 of these oocytes mature and are released in a woman’s lifetime.2
During a female’s reproductive years, the granulosa cells become cuboidal and increase in number. Gap junctions develop between the granulosa cells and between the oocyte and the granulosa cells.
The oocyte secretes an acellular coat that surrounds it. This is known as the zona pellucida and is important during fertilisation. It contains three proteins: ZP 1, 2, and 3. Sperm recognises ZP 3, and subsequent changes in the remaining proteins harden the zona pellucida, preventing the penetration of more sperm cells.
Up until this point, the development of these follicles has been gonadotrophin-independent, but for continued follicular development, gonadotrophs are needed. A dominant follicle must be selected to undergo ovulation (release from the ovaries to be fertilised by a sperm in the fallopian tubes). The process of selecting this dominant follicle starts 3 months before that specific follicle is ovulated. Of the thousands of follicles available, only a small cohort of 3-11 undergo further changes each month.4
What are gonadotrophs?
Gonadotrophs are a type of peptide hormone, meaning they are water-soluble and act on receptors found on cell surfaces. There are two types of gonadotroph hormones: Luteinising Hormone (LH) and Follicle follicle-stimulating hormone (FSH).
Following puberty, the arcuate nucleus of the hypothalamus produces and releases gonadotropin-releasing hormone (GnRH) in a regular pulsatile fashion. GnRH stimulates the gonadotroph cells of the anterior pituitary to release LH and FSH.
FSH causes an increase in the number of granulosa cells. These granulosa cells differentiate into two distinct areas: the theca interna and the theca externa. This is known as a secondary follicle.
Tertiary follicles (also called antral follicles) are formed when antrum (fluid filled spaces) appear. The antrum contain plasma as well as factors secreted by the granulosa cells (e.g. oestrogen and growth factors).
As more fluid builds up and the antrum enlarges, the developing follicle is known as a graafian follicle.
Which graafian follicle is chosen for ovulation?
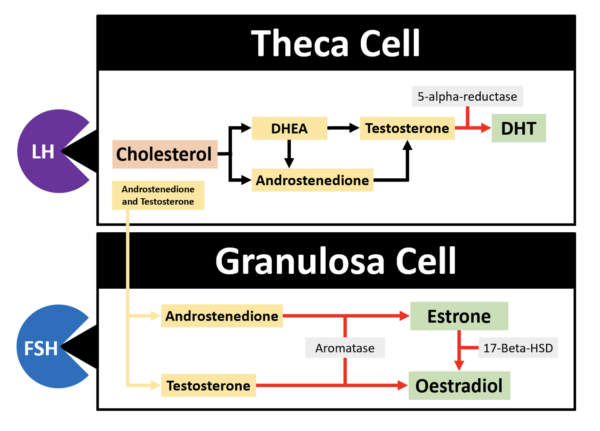
LH binds to the theca cells of the developing follicles, stimulating the production of androstenedione and testosterone, using cholesterol as a precursor. This process is complex and is described in the image below. A precursor of these hormones is dehydroepiandrosterone (DHEA), which is considered a pre-hormone alongside androstenedione because they can both be converted to testosterone or oestrogen, which in turn elicit an effect.
FSH binds to the granulosa cells to stimulate the production of aromatase. Androstenedione and testosterone diffuse out of the theca cells and into the granulosa cells and are converted by aromatase (via aromatisation) to 17 beta-oestradiol.
As the follicles grow, this type of oestrogen increases, acting as a negative feedback signal on the anterior pituitary, decreasing the release of FSH and LH.
FSH also stimulates granulosa cells to release inhibin B, which blunts FSH secretions in the late follicular phase.
With less FSH in the blood, there is only enough to stimulate one follicle. This dominant follicle will have the most FSH receptors, allowing it to grow quickly. FSH works with insulin like growth factor and vascular endothelial growth factor to aid in developing the dominant follicle.
What happens to the non-dominant follicles?
Low levels of androgens (produced in the theca cells due to LH stimulation) encourage aromatisation. However, aromatisation (in the ovary) can only occur if FSH is present. Therefore, if a follicle lacks enough FSH receptors for the androgens to be aromatised to oestrogens, the testosterone is converted (by 5-alpha-reductase) to dihydrotestosterone (DHT).
DHT inhibits aromatisation, cannot be aromatised, and inhibits FSH induction of LH receptor expression on the granulosa cells surrounding the antrums. Yes, LH receptors are also found on granulosa cells. LH acts on these receptors, stimulating the production of a small amount of progesterone to prepare the follicle for luteinisation (development of the corpus luteum, which occurs after ovulation). As a result, the non-dominant follicles disintegrate, a process known as atresia. This will also occur naturally in roughly 1,000 primary follicles each month.
How does the LH surge occur?
As the dominant follicle produces more and more oestrogen, the negative feedback effect on LH production becomes a positive feedback effect, and the LH surge is initiated.
LH causes the oocyte to re-enter miosis in preparation for fertilisation and stimulates the release of the oocyte from the dominant follicle (ovulation). This occurs because LH increases the levels of prostaglandins in the follicular fluid, which causes smooth muscle contraction in the ovary, and LH increases the expression of proteolytic enzymes in the follicle, which helps to thin the follicular wall, allowing the oocyte to pop out. The LH surge lasts 48 hours.
As inhibin decreases at the end of the luteal phase, the start of the next cycle will have low levels of all hormones except FSH. A new dominant follicle is recruited, and the cycle continues.
Types of oestrogen2
Estrone (E1):
- The main oestrogen in postmenopausal women
- Converted from androgens in peripheral tissues
- Attempts to maintain oestrogenic effects in the absence of ovarian activity
Oestradiol (E2):
- The most potent oestrogen produced by the ovaries in premenopausal women
- Maintains the menstrual cycle
Estriol (E3):
- Weakest of all the oestrogens
- Produced by the placenta during pregnancy
- Maintains the uterus lining and supports foetal development
Perimenopause
Perimenopause (also known as climacteric or menopausal transition) is defined as a period of menstrual cycle irregularity extending until one year after the permanent cessation of the menses.
Perimenopause usually occurs between the ages of 40 and 50. The average age of onset is 47 and can last between 4-8 years.4
The number of oocytes decreases with age (via the process of atresia), and because of this, the amount of inhibin released by the granulosa cells decreases.
Less inhibin results in more FSH released. An increase in FSH also increases the activity of the aromatase enzyme.6 As a result, levels of oestradiol fluctuate, and concentrations can be higher than they were before the age of 35.
Oestrogen levels only start to decrease about one year before menopause, as the small number of oocytes left can no longer make up for the effect that high FSH levels provide.
Menstrual cycles become more irregular, with ovulation occurring less, not only because the number of oocytes has decreased but also because, with age, the quality of the oocytes also decreases.
Postmenopause
Postmenopause is defined as the time following the final menstrual period.
Post-menopause, there are no more oocytes. Levels of oestrogen decrease, leading to reduced negative feedback on the hypothalamus and pituitary gland, resulting in a 10-20-fold increase in the amount of FSH and a 3-fold increase in the amount of LH.
There is more FSH than LH because LH is cleared from the body faster than FSH (LH has a 20-minute half-life compared to FSH, which has a 4-hour half-life), and LH does not have an inhibin equivalent. After 3 years, these levels start to decrease as the cells of the pituitary gland age and lose their ability to respond to GnRH.2 Post menopause the ovary weighs approximately 10g but can still be seen via ultrasound.4
What is left of the ovaries is stromal tissue and remnant steroidogenic tissue from oocyte atresia. These tissues respond to the remaining GnRH, FSH, and LH by releasing testosterone. Because testosterone isn’t converted to oestrogen in the follicles, testosterone levels actually rise within the first years postmenopause (this is not seen in every woman).
Ultimately, testosterone levels post-menopause drop by 25%. 25% of testosterone in women comes from the ovaries, another 25% comes from the adrenal gland, and the remaining 50% comes from peripheral conversion of androstenedione and as little to no androstenedione is produced by the ovaries post menopause (circulating levels drop by 50%) the adrenal glands become the predominate source of androstenedione and testosterone, which can be converted in the peripheries (most notably in adipose tissue) to estrone.2
Estrone is the primary oestrogen type postmenopause. Levels premenopausal range from 30-200pg/mL, and levels post-menopause range from 30-70pg/mL. Levels of oestradiol decrease 20 times post-menopause.2
Levels of androstenedione and testosterone remain constant post-menopause. However, levels of DHEA produced by the adrenal glands slowly decrease, and by the age of 70-80, have dropped 74% from peak levels (peak levels are seen between the ages 20 and 30).4
Sex hormone-binding globulin (SHBG) production declines after menopause. SHBG is produced by the liver. Its production increases in hyperthyroidism, pregnancy, and oestrogen administration. Androgens, progestins, growth hormone, insulin, weight gain and corticoids lower SHBG levels.
Steroid hormones are bound in peripheral circulation to SHBG (as well as albumin, thyroid-binding globulin, and corticosteroid-binding globulin). Only 1% of steroid hormones are not bound to a carrier protein and are considered biologically active. A decrease in SHBG will result in higher numbers of unbound oestrogen and testosterone.4
Ultimately, postmenopause is a time of relative androgen excess, which can result in mild hirsutism.
Epidemiology of menopause
The Study of Women’s Health Across the Nation (SWAN) is an American study of women undergoing perimenopause. The median age of menopause was found to be 51.4.
There may be a genetic link to the age at which menopause occurs.
There is no reliable evidence to suggest that the age at which a woman experiences her first period affects the age at which the same woman starts menopause.
Ethnic differences:5
- African American and Latina women start menopause 2 years earlier than white women.
- A Nigerian study found the average age of menopause to be the opposite, 2 years higher than that of white women.
- Asian and Caucasian women start menopause at a similar age, but Thai women have lower median age (49.5 years).
- India reports an average age of menopause up to 5 years earlier.
Why would menopause begin earlier:5
- Smoking can cause menopause to occur 1-2 years earlier. Perimenopause is also shorter. Cigarette smoke contains polycyclic aromatic hydrocarbons, which are toxic to the ovarian follicles. Drug metabolism is increased in smokers so circulating oestrogen may be reduced in smokers. On top of this, smoking also has anti-oestrogenic effects.
- A Papua New Guinea study found malnourished women start menopause 4 years before well-nourished women. Vegetarians also start menopause early, meat is known to increase the release of LH and FSH and the length of the menstrual cycle.
- Lower socioeconomic status.
Why would menopause begin later:
- Increased parity
- Prior use of oral contraceptives is debatable
- Alcohol
Physiology of menopausal symptoms
Symptoms of menopause can be split into 2 categories:
- Symptoms due to unopposed oestrogen
- Symptoms due to decreased levels of oestrogen
Oestrogen and progesterone are steroid hormones. They are lipophilic and stimulate receptors inside cells rather than on the surface. There are two types of oestrogen receptors (ER); ER alpha and ER beta, which are found in the nucleus of cells.
Oestrogen receptors are found in many tissues, so changes in oestrogen levels will have systemic effects. Oestrogen receptors are found in:
- Reproductive organs
- Bone tissue
- Brain tissue
- Cardiovascular tissue
- Adipose tissue
- Hepatic tissue
- Immune cells
Unopposed oestrogen is when the effects of oestrogen are not counteracted by progesterone. Progesterone blunts the expression of oestrogen receptors in tissues.
In the perimenopause, there is excess unopposed oestrogen due to:4
- Increased FSH, increasing aromatisation of androstenedione and testosterone.
- Less ovulation means a corpus luteum is not being formed, which is the primary source of progesterone.
- Decreased levels of Sex Hormone-binding Globulin, increasing the amount of free/ biologically active oestrogen.
- Obesity and increased adipose tissue increase peripheral aromatisation of androstenedione and testosterone.
Increased risk of endometrial cancer
Oestrogen stimulates the lining of the endometrium to proliferate. Progesterone normally keeps the proliferative effects of oestrogen in check.2
The increased level of unopposed oestrogen, along with obesity, is a significant risk factor for the development of endometrial cancer. This risk is highest from the age of 50-60 years old.
Sexual health
Vaginal atrophy
The vagina changes due to the decreased levels of oestrogen:2
- Collagen loss
- Adipose tissue loss
- Ability to retain water lost
- Walls shrink, and the rugae flatten
- The surface epithelium loses its outer fibrous layer
- Sebaceous gland secretions decrease
- Blood vessels in the wall narrow
- pH becomes more alkaline
These changes make the vagina prone to bleeding, especially after sex (dyspareunia), less flexible and dry. This can also lead to itching, irritation and burning.
The change in pH is not favourable for the lactobacillus that normally populates the vagina, increasing the risk of infections.
The weakening of the vaginal walls can increase the risk of stress incontinence and pelvic organ prolapse.2
Low libido
Lower testosterone levels decrease libido.
Oestrogen’s effect on the vagina (e.g. dryness) can lead to painful sex.
Bone health
Osteoporosis
Osteoporosis is low bone density, specifically having a bone density 2.5 standard deviations below the mean of an average healthy woman with peak bone mass (also known as the T score).
Trabecular bone is most affected by menopause, and 20% of bone density may be lost. Osteoporosis increases the risk of fractures, especially of the hip and vertebrae.7
To understand why the risk of osteoporosis increases after menopause, we must first understand the process of bone remodelling.
Two hormones, RANKL and Osteoprotegerin (OPG), regulate bone remodelling. Lining cells (a type of osteoblast that has stopped making new bone) respond to hormones and cytokines by releasing RANKL.
RANKL binds to osteoclasts, which then proliferate and secrete acid and proteases, which create a pit in the bone. Osteoblasts come along and deposit collagen to be mineralised, but they also release OPG, which inhibits osteoclasts from destroying any more bone.
Oestrogen normally encourages the release of OPG, but as levels decrease after menopause, less OPG is released and less osteoclasts are inhibited from destroying bone.6
Dental health
Oestrogen also plays an important role in dental health. Osteoporosis of the alveolar bone, accompanied by decreased salivary secretions, increases the risk of tooth loss.
Complications of poor dental health due to a lack of oestrogen include:
- Gingivitis (inflammation of the gums)
- Periodontitis (inflammation of the periodontium, the soft tissue and bone which supports the teeth)
- Toothache
- Halitosis
- Dry mouth
Vasomotor symptoms
Vasomotor refers to the regulation of blood vessel diameters. A “hot flush” is a sudden reddening of the skin over the face, neck, and chest, with associated tachycardia, hypertension, and the feeling of a raised body temperature, lasting 1-5 minutes. It is most common in warm environments, after eating spicy food and at night, often waking women up in sweats.2
Hot flushes can begin an average of two years before menopause. 85% of women will continue to have hot flushes longer than one year. Of these women:
- 25-50% will have hot flushes for 5 years
- 15% will have hot flushes for more than 15 years
The physiology is not completely understood. The common consensus is that hot flushes are caused by rapid oestrogen withdrawal or level fluctuations rather than chronically low oestrogen. This makes sense as women with Turner’s syndrome, who have no/non-functional gonadal tissue, have chronically low oestrogen without vasomotor symptoms.4
The hypothalamus is responsible for temperature regulation. Women with hot flushes have a narrower zone of temperature regulation, meaning smaller temperature changes can result in abnormal compensatory responses.2
As the body temperature rises, peripheral vasodilation increases skin temperature and heart rate. The increase in cardiac output, along with the sympathetic nervous system activation, increases blood pressure despite vasodilation being the predominant physiological outcome. This unwarranted response to such a small increase in temperature leads to an eventual reduction in core body temperature.6
Oestrogen affects the concentrations of various neurotransmitters in the brain. A decrease in oestrogen reduces α2-adrenergic receptor concentrations in the hypothalamus. When activated, these neurones inhibit noradrenaline release; therefore, there are fewer inhibitory receptors and more noradrenaline. Noradrenaline is one of the neurotransmitters responsible for the effects of a hot flush.
A decline in oestrogen is also associated with a decline in serotonin levels, another neurotransmitter found in the thermoregulatory centre of the hypothalamus.8
Changes in the distribution of adipose tissue
Adipose tissue is found subcutaneously (under the skin) and viscerally (around internal organs in the abdominal cavity). Visceral fat is more hormonally active and pro-inflammatory than subcutaneous fat; it can release adipokines and cytokines, directly influencing insulin sensitivity. Therefore, increased visceral fat (especially around the liver) is a risk factor for insulin resistance and cardiovascular disease.9,10
The distribution of fat is different in males and females due to the effect sex hormones have on the regulation of preadipocyte differentiation, proliferation and adipogenesis.
Women have a higher percentage of body fat than men, which in premenopausal women, is composed of mostly subcutaneous white adipose tissue throughout the lower body, hips, and thighs. Postmenopausal women have a different phenotype due to decreased oestrogen levels. They have increased amounts of visceral fat and decreased amounts of subcutaneous fat.
When oestrogen binds to ER alpha on types of adipose tissue, these are the following effects:10
- Increased accumulation of subcutaneous fat
- Hyperplasia of subcutaneous fat, rather than hypertrophy, which is what is associated with obesity
- Insulin sensitivity
- Anti-inflammatory effects on visceral fat
After menopause, there are lower levels of oestrogen and, as a result, lower levels of ER alpha. So now the reduced stimulation of ER alpha results in:10
- Hypoxia and fibrosis of subcutaneous fat
- Hypertrophy and fibrosis of visceral fat, which is pro-inflammatory
- Insulin resistance
Neurological symptoms
Brain fog
Oestrogen regulates glucose transport, increases aerobic glycolysis, increases ATP generation, and provides an antioxidant effect within the brain.
Post menopause, low oestrogen levels in the brain means lower brain glucose levels. The brain uses fat from the myelin around nerves as its alternate fuel source. The breakdown of the myelin leaves debris behind, leading to inflammation.
An increase in oxidative stress also increases the amount of reactive oxygen species formed, whose effects would normally be dampened by oestrogen’s antioxidant ability.11,12
Brain function is affected in many areas, including the following:
- The brain stem (insomnia)
- The amygdala, the emotional centre of the brain (mood swings)
- The hippocampus, where memories are stored (memory loss)
A combination of these symptoms may produce the effect known as “brain fog”.11,12
Alzheimer’s disease
Lower oestrogen levels are associated with the build-up of abnormal amyloid-beta proteins, which are known to cause Alzheimer’s disease.11,12
Depression
The exact pathogenesis of depression is not known.
Low levels of oestrogen result in lower levels of serotonin, which in turn is associated with decreased mood.5 Oestrogen modulates other neurotransmitters (e.g. noradrenaline and dopamine).
DHEA and DHEAS are regulators of serotonin and GABA signalling. Levels of DHEAS are lower in depressed postmenopausal women compared to non-depressed post-menopausal women.
Other symptoms of menopause may contribute to depression.
Gastrointestinal symptoms
Abdominal pain and bloating
Oestrogen normally encourages gut motility.
Dyspepsia
Fluctuating oestrogen levels can affect stomach acid production. Menopause is a time of stress, increasing blood cortisol levels and stomach acid production.
Skin
In the skin, oestrogen encourages collagen production and helps to maintain the water content. Low oestrogen levels are associated with dry and wrinkled skin, which easily bruises.5
Cardiovascular health
Before the age of 40, men have double the risk of developing cardiovascular disease than women do. After menopause, this statistic evens out.2
Why are women protected from cardiovascular disease before menopause?13,14,15,16,17
Pre-menopause, women have high levels of high-density lipoprotein (HDL) and lower levels of low-density lipoprotein (LDL).
A lipoprotein comprises an outer layer of phospholipids and apoproteins surrounding an inner core of cholesterol and triglycerides.
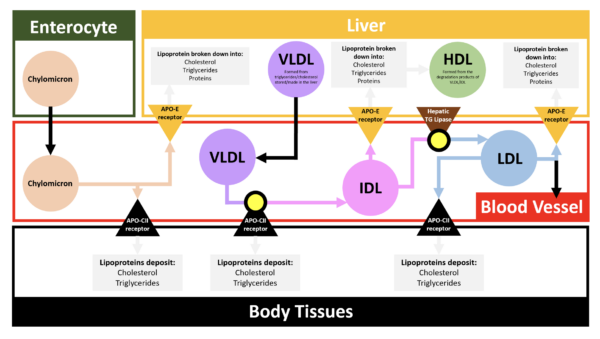
LDL contains high amounts of cholesteryl ester (the active form of cholesterol). A precursor lipoprotein to LDL, known as Very-Low Density Lipoprotein (VLDL) is made in the liver and its function is to transport cholesterol and triglycerides to body tissues.
After losing a certain amount of its content, it becomes intermediate-density lipoprotein (IDL), which returns to the liver, where it becomes HDL or is converted by hepatic lipases to LDL.
LDL can then do one of three things:
- 60% is returned to the liver, and its contents are recycled
- 40% stays in the blood to transport cholesterol to body tissues
- LDL in the blood may be deposited in the damaged endothelium of blood vessels, leading to atherosclerosis
HDL is produced in the liver from the degradation products of IDL and has the opposite function of LDL. It collects cholesterol from body tissues and returns it to the liver.
HDL has an important role in reducing atherosclerosis and, therefore, clot formation by removing cholesterol that has built up in the endothelium of blood vessel walls (see below).
Oestrogen’s positive effects:
- It suppresses the hepatic lipase enzyme, which converts IDL to LDL. As a result, more IDL is converted to HDL.
- It has antioxidant effects. It inhibits the oxidation of LDL, which is deposited in the endothelium. This oxidation is an important step in the inflammatory process of atherosclerosis (see below).
- It has vasodilatory effects by increasing endothelial production of nitrous oxide.
Oestrogen’s negative effects:
- Oestrogen increases the synthesis and activity of matrix metalloproteinases, which are produced by inflammatory cells and break down the protective fibrous cap of the atherosclerotic plaque, exposing the underlying thrombogenic collagen.
It is important to reduce the risk factors for atherosclerosis before menopause because when the time comes, and exogenous oestrogen is needed, the risk might outweigh the benefit if there are pre-existing plaques.
Exogenous oestrogens
Oral oestrogen is metabolised by the liver.2 Oestrogen metabolism in the liver can impact the hepatic synthesis of proteins. It can increase the production of clotting factors VIII, X, XII and XIII.17
Atherosclerosis
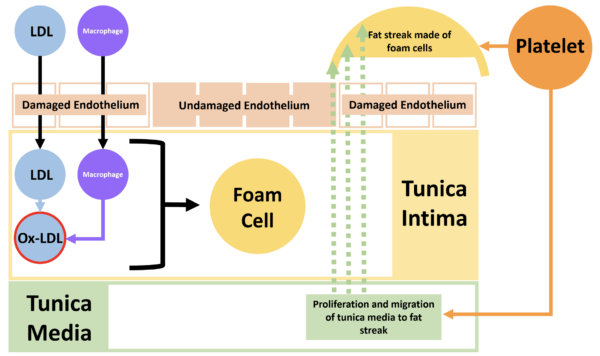
The endothelium of a blood vessel wall becomes damaged, allowing LDL to enter the tunica intima. An inflammatory reaction begins, and the endothelium releases ROS, which oxidises LDL into Ox-LDL, worsening the inflammatory process.
Damaged endothelium releases cytokines to attract monocytes. Monocytes enter the tunica intima, differentiate into macrophages, and engulf the Ox-LDL. If a macrophage takes up too much Ox-LDL it becomes a foam cell, which dies, releasing its content only for the cycle to continue and another macrophage to engulf the leftovers.
Luckily, HDL can come in and save the day. By binding to specific scavenger receptors on the surface of the macrophages, it can remove cholesterol and prevent macrophage apoptosis.
However, a problem can occur when HDL cannot enter the tunica intima. This happens when there are too many foam cells, which form a fat streak. This fat streak is thrombogenic.
Platelets arrive on the scene and release growth factors encouraging smooth muscle proliferation and migration from the tunica media to form a fibrous cap over the foam cells. Calcium is deposited and crystallised, stiffening the artery wall. If this fibrous cap breaks, a very thrombogenic surface is exposed to platelets and clotting factors, resulting in a thrombus or an embolism.
Premature ovarian insufficiency6
Premature ovarian insufficiency is defined as hypergonadotropic hypogonadism and amenorrhoea before the age of 40. It occurs in 1% of women.
The cause is due to one of two reasons. Either there is a lack of follicles, or the follicles are present but non-functional. As a result, oestrogen levels are decreased, and FSH levels are increased.
A diagnosis can be made in women under the age of 40 if she has menopausal symptoms or elevated FSH levels (more than 30IU/L) on two blood samples taken 4-6 weeks apart. A pelvic exam should not be routinely offered.
Causes:
- Turner syndrome: causes increased atresia of primordial follicles during the gestational period.
- Fragile X syndrome
- Galactosemia: galactose metabolites are toxic to the ovaries.
- FSH receptor mutations
- Ovarian toxins: mumps, cytomegalovirus, or tuberculosis may cause oophoritis; chemotherapy.
- Congenital adrenal hyperplasia
- Mutations affecting the binding of FSH and LH to receptors.
Differential diagnoses
Differential diagnoses of menopause can be split into:18
- Alternate diagnoses to the state of permanent secondary amenorrhoea, which is guaranteed with menopause
- Alternative diagnoses to the possible symptoms of menopause
Secondary amenorrhoea
Other causes of secondary amenorrhea include:
- Pregnancy
- Hormonal contraception
- Thyroid pathology
- Stress
- Pituitary prolactinoma – prolactin prevents the release of GnRH
- Pituitary failure – radiotherapy or Sheehan’s syndrome
- Polycystic ovarian syndrome
- Asherman’s syndrome
Menopause symptoms
Irregular vaginal bleeding
- Endometrial polyps
- Uterine fibroids
- Adenomyosis
- Endometrial hyperplasia/cancer
- Vulval, vagina or cervical lesions
Sleep changes
- Normal aging
Vaginal atrophy
- Trauma
- Infection
- Lichen sclerosis
Urinary incontinence
- Obesity
- Gynaecological surgery
- Multiparity
Weight gain
- Lifestyle
Loss of libido
- Insomnia
- Depression
- Androgen deficiency
Joint pain
Skin changes
- Ageing
- Smoking
- Sun exposure
Hot flushes
- Hyperthyroidism
- Pheochromocytoma
- Carcinoid syndrome
- Pancreatic cancer
- Renal cell cancer
- Paraneoplastic syndrome
- Excess alcohol
- Lymphoma
- Tuberculosis
- Anxiety
- Dumping syndrome
- Food additives (e.g. monosodium glutamate and sulphites)
- Drugs (e.g. opiates, nitrates, SSRIs, calcium channel blockers, GnRH agonists)
Reviewer
Dr Rafea Khan MBBS, MRCOG (2009), MRCGP
GP with specialist interest in Obstetrics and Gynecology
Roundwell Medical Centre
References
- Online Etymology Dictionary. Menopause. August 2020. Available from: [LINK]
- Hugh S. Taylor, Lubna Pal, Emre Seli. Speroff’s Clinical Gynecologic Endocrinology and Infertility Ninth Edition. 2020.
- Andrew Baldwin. Oxford Handbook of Clinical Specialties 11th Edition. 2020.
- Hoffman. Williams Gynaecology Forth Edition. 2020.
- Rogerio A. Lobo. Menopause: Biology and Pathobiology. 2000.
- McMaster Pathophysiology Review. Menopause. February 2013. Available from: [LINK]
- Medscape. Menopause. May 2023. Available from: [LINK]
- Robert R. Freedman. Physiology of Hot Flashes. 2001. Available from: [LINK]
- National Library of Medicine. Adipose Tissue: Physiology to Metabolic Dysfunction. April 2020. Available from: [LINK]
- Frontiers. The Regulation of Adipose Tissue Health by Estrogens. May 2022. Available from: [LINK]
- National Library of Medicine. Estrogen: a master regulator of bioenergetic systems in the brain and body. August 2013. Available from: [LINK]
- Maki, P. M., & Thurston, R. C. (2020). Menopause and brain health: hormonal changes are only part of the story. Front Neurol. 2020; 11: 562275.
- Derek G. Waller. Medical Pharmacology and Therapeutics sixth edition. 2022.
- Robbins & Cotran. Pathological Basis of Disease Tenth Edition. 2021.
- Peter Ronner. Nettter’s Essential Biochemistry. 2018.
- John Hall. Guyton and Hall Textbook of Medical Physiology Fourteenth Edition. 2021.
- AHA Journals. Female Hormones and Thrombosis. February 2002. Available from: [LINK]
- NICE. Menopause: What else might be causing symptoms? September 2022. Available from: [LINK]