- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Renal replacement therapy (RRT) refers to life-supporting treatments for severe acute kidney injury (AKI) or end stage kidney disease.1
Haemodialysis (HD) is a form of RRT that filters the blood using an external filter, a dialyser. It has two main forms of administration:2
- Conventional HD is a type of intermittent RRT that usually involves four hours per session, three times per week
- Continuous HD involves fluid and solute clearance over 24 hours and is used almost exclusively for acute RRT in critical care settings
Indications for RRT
Acute RRT
Important indications can be remembered using the mnemonic AEIOU:3,4,5
- Acidosis: severe metabolic acidosis, pH <7.2
- Electrolyte disturbance: severe, refractory hyperkalaemia, >7.0
- Ingested toxins: BLAST (barbiturates, lithium, alcohol, salicylate, theophylline)
- Refractory pulmonary oedema
- Uraemia: manifesting as pericarditis/encephalopathy
Chronic RRT
NICE recommends the initiation of chronic RRT when there is the presence of:1
- Symptomatic uraemia (pericarditis/encephalopathy)
- Biochemical measures (electrolyte or acid-base disturbances that are refractory to medical therapy) or uncontrollable fluid overload
- Asymptomatic with an eGFR of 5-7 ml/min/1.73m2
Other indications outlined by Kidney Disease Improving Global Outcomes (KDIGO) include:5
- Anorexia
- Reduced energy level
- Weight loss with no other potential explanation
- Progressive deterioration in nutritional status that is refractory to interventions
Types of dialysis
Dialysis is a form of RRT which replaces the kidney’s role in filtering blood to maintain homeostasis. Its primary goal is to remove excess water and uraemic wastes.6,7 It also removes excess acid, regulates electrolyte levels, and eliminate metabolic waste products.
However, dialysis cannot replace the kidney’s ability to produce EPO (erythropoietin) and activate vitamin D via 1 alpha-hydroxylase.6,7
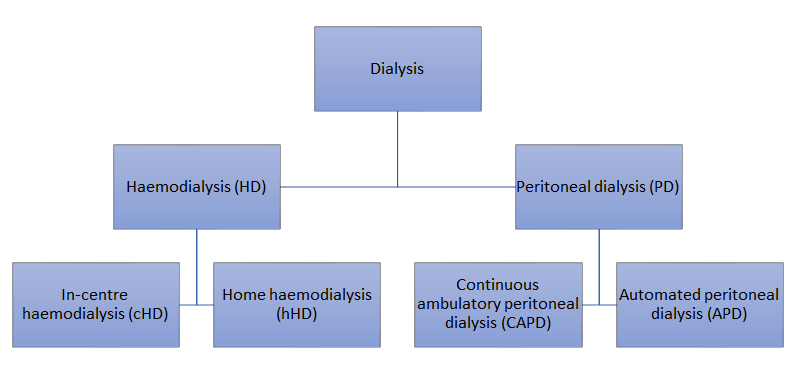
Choice of dialysis modalities
All patients who are likely to require RRT should be offered a choice of RRT or conservative management (which involves supportive management and controlling symptoms but without RRT).1,5
NICE does not provide a straightforward recommendation in the initial choice of dialysis modality, apart from recommending peritoneal dialysis (PD) as the first choice in patients who are two years or younger.1
The choice of dialysis modality should reflect a shared decision made between the clinician and patient after all available options are presented and explained to them.
Vascular access in haemodialysis
Access in haemodialysis refers to forming an artificial connection between the patient’s circulatory system and the dialysis machine. The most common options are arteriovenous access and central venous catheter access.
Arteriovenous access
Arteriovenous access is indicated in long-term dialysis, which involves surgical anastomoses between an artery and a vein.5,8 It is most commonly formed in the non-dominant arm between the radial artery and the cephalic vein, called a radio-cephalic fistula.8 Other possibilities include brachio-cephalic fistula and brachio-basilic fistula.
There are two main types of arteriovenous access:5,8
- Arteriovenous fistula (AVF): an artery is directly connected to a vein. It is considered the optimal form of vascular access for HD. An AVF takes at least six to eight weeks to mature, allowing the thickening of the vein’s wall to withstand arterial pressure. NICE recommends establishing an AV fistula six months before the anticipated initiation of dialysis.
- Arteriovenous graft (AVG): a synthetic or biological graft is used to connect an artery to a vein. The most common type of graft is polytetrafluoroethylene (PTEF). Unlike AVF, it can be used within days (PTEF grafts can often be used within two weeks). However, it is at greater risk of developing thrombosis and infection and has a shorter life than AVF.
Care of a fistula
Dialysis access is extremely precious, and precautions must be taken to preserve and care for the fistula.
Precautions during HD include:8
- Needles should only be inserted by a trained operator
- Avoid needling the same site repetitively, as it increases the risk of a false aneurysm (pseudoaneurysm) formation
General precautions include::8
- Do not perform venepuncture from a fistula
- Do not perform intravenous cannulation between the elbow and wrist
- Do not apply a blood pressure cuff or tourniquet on the arm with a fistula
For more information on examining a fistula, see the Geeky Medics guide to performing a renal system examination.
Importantly, the absence of thrill on palpation and the absence of bruit on auscultation suggests thrombosis.
Patients should be educated to examine the fistula, and swift intervention within 48 hours via either interventional radiology (local thrombolysis) or surgery is warranted to salvage the fistula.8
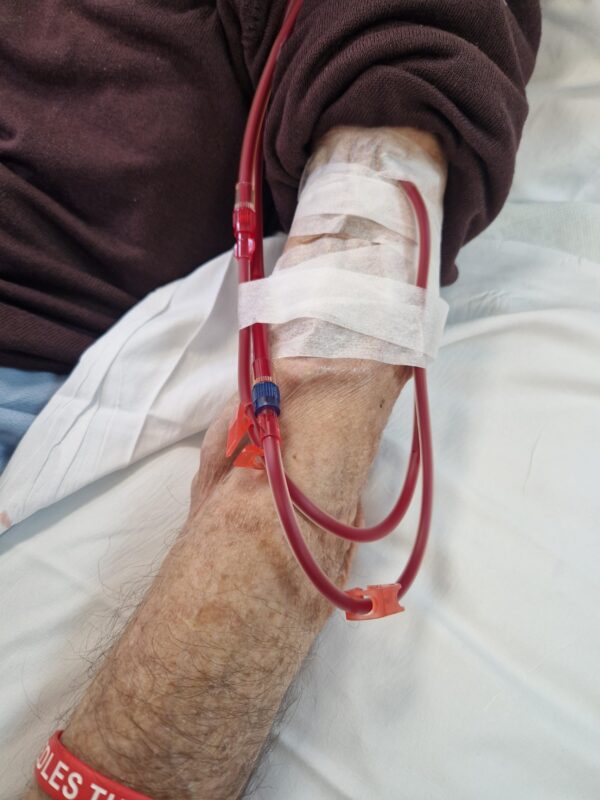
Central venous catheter (CVC) access
Catheters used in HD have two openings, one to draw blood from the veins to the dialysis circuit (red), one to drain dialysed blood back to the patient (blue).
Two main types of CVCs are used to obtain central venous access in HD:8,9
- Temporary dialysis catheter: allows immediate use when emergency dialysis is indicated in AKI. Potential sites for catheter insertion include the internal jugular, subclavian and femoral veins. Due to the much higher risk of infection, they should only be left in situ for less than two weeks.
- Tunnelled haemodialysis catheter: a large bore, double-lumen venous catheter is inserted in a central vein and is ‘tunnelled’ subcutaneously helping to reduce the risk of infection. A classic indication for the use of tunnelled catheter is while waiting for a fistula to mature.
Choosing haemodialysis access
KDIGO recommends that the choice of HD access should primarily depend on the likelihood of long-term survival, defined by more than one year of survival:5
- If long-term survival is likely to be more than one year, an AVF is preferred. If creating an AVF is not feasible, a cuffed tunnelled haemodialysis catheter should be considered.
- If long-term survival is likely to be less than one year, AVG or CVC are more appropriate unless opting for conservative management without RRT.
Principles of HD
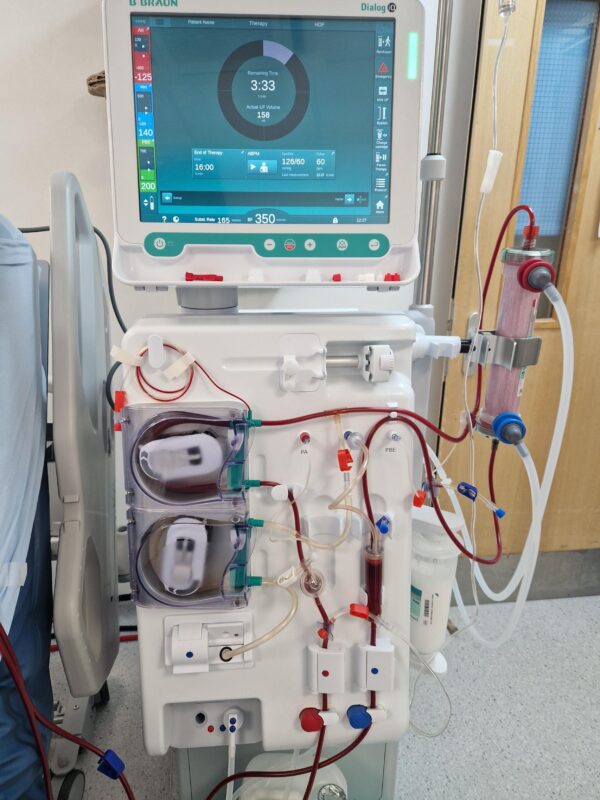
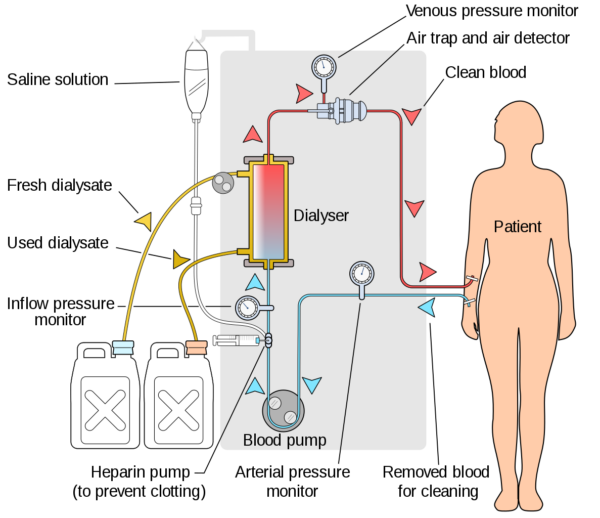
The HD apparatus is made up of the blood circuit and the dialysis solution circuit that is bridged by a dialyser, where artificial filtration of the blood takes place.
The HD circuit is described below:
- A pump removes blood from the patient to the machine
- Blood is anticoagulated with heparin to prevent clotting
- Blood is pumped through a dialyser where filtering of the blood takes place (see below for more details)
- Filtered blood is returned to the patient, while used dialysate is discarded
Throughout the circuit, the blood pressure is being monitored. Importantly, a post-pump pressure monitor helps detect impending clotting within the circuits. A venous air trap and air detector is also present to ensure that no air enters the individual circulation and causing an air embolism.8
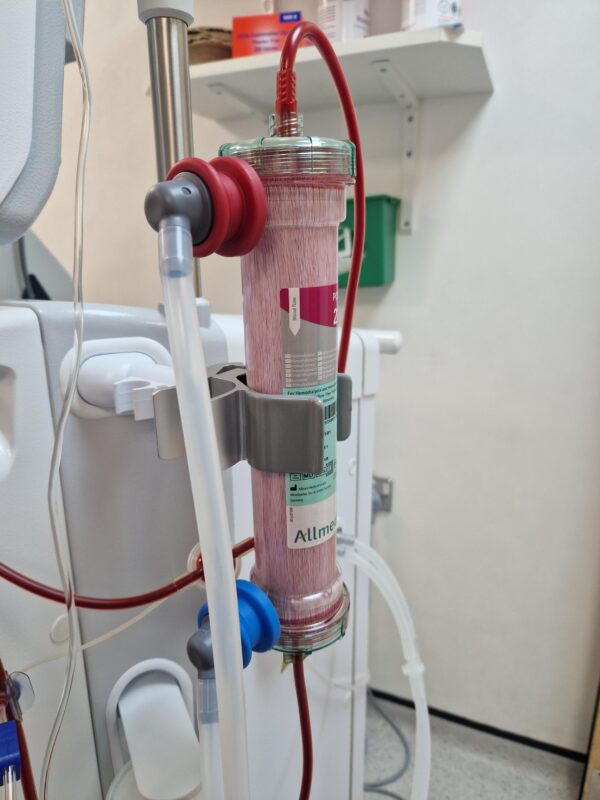
How does the dialyser filter blood?
In HD, the dialyser filters the blood. It contains two compartments, one for blood and one for dialysate, separated by a semi-permeable membrane.
The dialysate is a solution of purified water containing sodium, potassium, calcium, magnesium, chloride, dextrose, and a buffer (usually bicarbonate).8
In the dialyser, excess water and unwanted solutes passively diffuse across the semi-permeable membrane into the dialysate fluid down a concentration gradient. The concentration gradient is maintained by continuously replacing the dialysate.
Only water and low-molecular-weight solutes can pass through the semi-permeable membrane, so blood cells and other intravascular proteins are not lost. Blood and dialysate flow in opposite directions to achieve counter-current flow, which maximises the concentration gradient and the efficacy of dialysis.
Haemodialysis vs haemofiltration
Haemodialysis removes solute by diffusion (driven by a concentration gradient) and requires dialysate. It is less effective at removing larger molecular weight solutes (>20kDa).8
Haemofiltration removes solute by convection (driven by a positive transmembrane pressure), and no dialysate is required.8 Since large volumes are filtered, replacement fluid is given pre or post-filter to avoid hypovolaemia. This allows large molecules to be removed efficiently.
Continuous haemofiltration is associated with greater haemodynamic stability and is the preferred RRT modality in a critical care setting.8
Assessing dialysis adequacy in HD
Kt/V is a measurement of urea clearance and is considered the most clinically valid measurement of dialysis adequacy and dose.10
The Renal Association clinical practice guideline for haemodialysis recommends:10
- A minimum of 12 hours of HD per week
- Monitoring of dialysis dose on a monthly basis
- Target dialysis dose should consistently achieve a minimum Kt/V of 1.2
The above points apply for thrice weekly patients, the conventional HD regimen.
Contraindications
The only absolute contraindications for maintenance HD are:5
- Absence of possible vascular access
- Haemodynamic instability
Some relative contraindications include coagulopathy and needle phobia.7
Complications
Medical emergencies
Some reactions are considered medical emergencies where prompt recognition and immediate cessation of dialysis is warranted:7,11,12
- Dialysis disequilibrium syndrome: acute cerebral oedema secondary to the rapid shifting of mainly urea from the blood. To prevent this, consider starting with a shorter first session, use a smaller dialyser, and reduce the blood flow rate to slow down the rate of urea clearance.
- Dialyser reactions: interaction between blood components and the HD machine semi-permeable membrane. It could either be IgE-mediated (type A), causing an anaphylactic-like presentation, or anaphylactoid/non-IgE-mediated (type B), causing back and/or chest pain that is usually self-limiting.
- Acute intravascular haemolysis: can be caused by chemical reactions (e.g. contamination of dialysate by chlorine, copper, zinc, chloramine, formaldehyde etc.) or mechanical forces (e.g. kinking of the lines, overheating of the dialysate fluid).
- Air embolism
- Haemorrhage from vascular access
Intra-dialytic complications
The following are some common complications one may experience during HD:7,11,12
- Hypotension can occur as blood is being pumped out of the circulation at a rate of 300-500 mL/min and is irrespective of the cardiac cycle. Risk is increased in patients with high interdialytic weight gains.
- Muscle cramps, especially in the lower limbs. The exact pathogenesis is unknown, it is thought to be caused by muscle hypoperfusion with secondary impaired muscle relaxation.
A range of non-specific features like headache (one of the most reported symptoms), vomiting, chest pain, back pain and pruritus are common for individuals to experience during HD.13
Long-term complications
Dialysis-related complications7,11,12
- Increased risk of bleeding: caused by platelet dysfunction due to CKD and/or platelet contact with the dialysis membrane.
- Electrolyte disturbances: hyperkalaemia is the most common, as well as hyponatraemia, hypocalcaemia, and hyperphosphataemia. These may give rise to fatal cardiac arrhythmias.
- CKD-MBD (chronic kidney disease-mineral bone disorder)
- Acquired cystic kidney disease
- Dialysis-related amyloidosis
Vascular access-related complications7,11,12
- Loss of access due to thrombosis or central venous stenosis
- Catheter-related bacteraemia
- Local aneurysm or pseudoaneurysm
- Dialysis access-associated steal syndrome: distal limb ischaemia secondary to the AV access shunting blood away from the limb
- Dialysis vascular access haemorrhage
Cardiovascular disease
The risk of cardiovascular disease is 10 to 20 times higher in patients undergoing dialysis than in normal people; it is the leading cause of death in these patients:6,14-16
- Inflammation in the kidneys and the process of dialysis also affect endothelial function, aggravating the risk of hypertension and cardiovascular diseases.
- An immunological response involving macrophages and granulocytes occurs against the artificial dialysis membrane, resulting in increased free radical production and oxidative stress.
- Interleukin-1 inhibits the peripheral conversion of T4 to T3, where low T3 levels contribute to left ventricular hypertrophy.
Non-specific long-term complications11
- Insomnia
- Bone and joint pain
- Restless leg syndrome
- Loss of libido and erectile dysfunction
- Dry mouth
- Anxiety
Key points
- Haemodialysis is a form of renal replacement therapy that artificially filters blood to maintain homeostasis.
- The conventional haemodialysis schedule involves a four-hour session three times a week.
- The ideal vascular access for haemodialysis is an arteriovenous fistula, but it takes six to eight weeks to mature before being used.
- Hemodialysis utilises passive diffusion and counter-current flow mechanisms to filter blood.
- Headaches, muscle cramps, and hypotension are common side effects that one might experience during haemodialysis.
- Cardiovascular disease is the leading cause of death in patients who are on long-term dialysis.
Reviewers
Dr Roberta Callus
Consultant Nephrologist
Deborah Grove
Consultant Nurse for Renal Medicine
Editor
Dr Jess Speller
References
- National Institute for Health and Care Excellence (NICE). Renal replacement therapy and conservative management (Clinical guideline [NG107]). NICE 2018. Published 2018 Oct. Available from: [LINK]
- Pauly RP. Survival comparison between intensive hemodialysis and transplantation in the context of the existing literature surrounding nocturnal and short-daily hemodialysis. Nephrology Dialysis Transplantation 2013 Jan;28(1):44-7.
- Malhotra A. Acute Kidney Injury (AKI). Geriatric Trauma and Acute Care Surgery 2017 Jul;367-380.
- Baker JB, Navarro Y, Sisroe TA, Everett C. Indications for Urgent and Emergent Hemodialysis. Annals of Vascular Surgery 2024 Jan;98:39-40.
- Chan CT, et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International 2019 Jul;96(1):37-47.
- Vadakedath S, Kandi Venkataramana. Dialysis: A Review of the Mechanisms Underlying Complications in the Management of Chronic Renal Failure. Cureus 2017 Aug;9(8):e1603.
- Murdeshwar HN, Anjum F. Hemodialysis. Treasure Island (FL): StatPearls Publishing 2024 Jan [Internet]. Updated: 2023 Apr. Available from: [LINK]
- Steddon, S. et al. Chapter 4 Dialysis. Oxford Handbook of Nephrology and Hypertension. Oxford: Oxford University Press, 2018.
- Hemodialysis Catheters: How to Keep Yours Working Well. National Kidney Foundation. 2018. Available from: [LINK]
- Ashby D, et al. Renal Association Clinical Practice Guideline Haemodialysis. The Renal Association. 2019 Jul. Available from: [LINK]
- NHS. Dialysis. Last reviewed: 2021 Sep. Available from: [LINK]
- Krause RS. Dialysis Complications of Chronic Renal Failure. Medscape. Last updated: 2023 Nov. Available from: [LINK]
- Headache attributed to disorder of homeostasis. The International Classification of Headache Disorders 3rd Available from: [LINK]
- Survival and Cause of Death in Adult Patients Receiving Renal Replacement Therapy. The Renal Association UK Renal Registry. Available from: [LINK]
- Bhandari SK, et al. Causes of Death in End-Stage Kidney Disease: Comparison between the United States Renal Data System and a Large Integrated Health Care System. American Journal of Nephrology 2022 Mar;53(1):32-40.
- Saravanan P, Davidson NC. Risk Assessment for Sudden Cardiac Death in Dialysis Patients. Circulation: Arrhythmia and Electrophysiology 2010 Oct;3(5):553-559.
Image references
- Figures 1 – 4. Deborah Grove
- Figure 5. YassineMrabet. A diagram of a haemodialysis circuit. License: [CC BY-SA]