- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Renal replacement therapy (RRT) refers to life-supporting treatments for severe acute kidney injury or end-stage kidney disease.1
Peritoneal dialysis (PD) is a form of RRT that utilises the patient’s peritoneal membrane (a double-fold membrane that lines the abdominal cavity and internal organs) as the interface between the blood and dialysate. It acts as a semi-permeable membrane to allow bidirectional movement of molecules of certain sizes across it down a concentration gradient.
There are two main types of PD:
- Continuous ambulatory peritoneal dialysis (CAPD)
- Automated peritoneal dialysis (APD)
Both allow the patient to carry out therapy at home after suitable training, depending on whether the patient prefers to dialyse during the day or the night. One should note that patients require PD daily.
PD cannot be used indefinitely. Normally, patients can remain on PD for up to eight years, although 20% of patients continue for more than ten years.2
It is important to educate patients that they will likely need to change to haemodialysis (HD) at some point unless they receive a kidney transplant.2
Indications for RRT
Acute RRT
Important indications can be remembered using the mnemonic AEIOU:3,4,5
- Acidosis: severe metabolic acidosis, pH <7.2
- Electrolyte disturbance: severe, refractory hyperkalaemia, >7.0
- Ingested toxins: BLAST (barbiturates, lithium, alcohol, salicylate, theophylline)
- Refractory pulmonary oedema
- Uraemia: manifesting as pericarditis/encephalopathy
Chronic RRT
NICE recommends the initiation of chronic RRT when there is the presence of:1
- Symptomatic uraemia (pericarditis/encephalopathy)
- Biochemical measures (electrolyte or acid-base disturbances that are refractory to medical therapy) or uncontrollable fluid overload
- Asymptomatic with an eGFR of 5-7 mL/min/1.73m2
Other indications outlined by KDIGO include:5
- Anorexia
- Reduced energy level
- Weight loss with no other potential explanation
- Progressive deterioration in nutritional status that is refractory to interventions
Dialysis
Dialysis is a form of RRT which replaces the kidney’s role in filtering blood to maintain homeostasis. Its primary goal is to remove excess water and uraemic wastes.6,7 It also removes excess acid, regulates electrolyte levels, and removes metabolic waste products.
However, dialysis cannot replace the kidney’s ability to produce EPO (erythropoietin) and activate vitamin D via 1 alpha-hydroxylase.6,7
The main dialysis modalities are expressed hierarchically below.
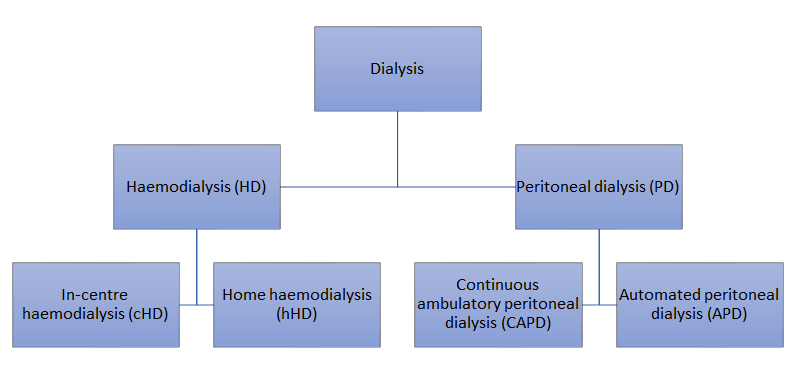
Choice of dialysis modalities
All patients who are likely to require RRT should be offered a choice of RRT or conservative management (which involves supportive management and controlling symptoms but without RRT).1,5
NICE does not provide a straightforward recommendation in the initial choice of dialysis modality, apart from recommending PD as the first choice in patients two years or younger.1
The choice of dialysis modality should reflect a shared decision made between the clinician and patient’s informed decision after all available options are presented and explained to them.
Peritoneal dialysis catheter placement
Before PD can be initiated, a catheter must be implanted into the peritoneal cavity to allow the bi-directional flow of dialysate. The most commonly used catheter is the Tenckhoff catheter, a flexible straight silicon tube with multiple pores on the intra-abdominal portion.8,9
Several techniques can be used to implant the catheter, including open surgical, laparoscopic, and percutaneous procedures. However, there is no strong evidence that favours one over another, as a range of patient and operator factors can affect the outcome.9,10
NICE recommends the creation of access around two weeks before the anticipated start of PD to prevent leakage.1
Principles of peritoneal dialysis
Each bag of dialysate is usually two litres in volume and contains an osmotic agent (traditionally glucose/amino acids/polypeptides), electrolytes (sodium, calcium and magnesium), and a buffer (lactate).11,12 Dialysate is introduced into the peritoneal cavity (dwell), where it encounters the peritoneal capillaries.
There are two main physiological concepts involved in the peritoneal transport:5,12
- Solute transport by diffusion, which occurs down a concentration gradient from the capillary blood into the dialysate.
- Ultrafiltration, the osmotic agent of dialysate generates a net positive intra-peritoneal osmotic gradient that moves excess water out of the capillary blood into the dialysate via aquaporins.
After a certain period of dwelling, the used dialysate is discarded.
Continuous ambulatory peritoneal dialysis (CAPD)
One cycle of CAPD is described below:
- Dialysate infused into the peritoneal cavity
- After the infusion, the operator disconnects the system and is free to perform their normal activities until the next exchange
- Dialysate dwells in the peritoneal cavity for four to six hours and filtration of the blood happens
- The operator re-connects the system and drains the used dialysate out of the peritoneal cavity
This cycle is usually repeated around four times a day. It is usually performed at home by the patient or assisted by a relative or carer.13,8
Automated peritoneal dialysis (APD)
APD works in the same way as CAPD, but instead of needing the patient to exchange the dialysate fluid manually, it is automated by a dialysis machine while the patient is asleep.13,14
The machine automatically infuses dialysate into the peritoneal cavity, leaving it to dwell, drain the fluid, and finally replace it with fresh dialysate. The cycle is usually repeated three to five times a night, and the patient usually needs to be attached to the machine for eight to ten hours per night.13,14
CAPD vs APD
Studies have failed to demonstrate significant differences between CAPD and APD regarding important clinical outcomes, including mortality.15,16,17
However, patients on APD have reported significant psychosocial advantages, such as being able to spend more time on work, family, and social activities. Therefore, APD may be considered advantageous in younger patient populations, especially those in education or employment.
There are cost differences between APD and CAPD. A comprehensive analysis in the United Kingdom has demonstrated that the annual direct cost per patient for APD was 24% more than CAPD (£20,295 vs £16,395).18
Assessing dialysis adequacy in PD
Both urea clearance (Kt/V) or creatine clearance (CrCl) can be used to monitor dialysis adequacy and should be measured at least six-monthly.19,20
A combined urinary and peritoneal urea clearance (Kt/V) of 1.7/week or a creatinine clearance (CrCl) of 50L/week/1.73m2 should be considered as the minimum treatment doses for adults.19,20
The peritoneal equilibration test (PET) should be used to monitor peritoneal membrane function. It is a measurement of solute clearance across the peritoneal membrane. Peritoneal membrane function should be monitored regularly, six weeks after initiating PD and at least annually or when clinically indicated.19,20
The most important predictor of survival in PD patients is not dialysis adequacy but the presence of residual renal function. Therefore, preserving residual renal function is vital:21
- Blood pressure control should be optimised
- Nephrotoxic drugs should be avoided
- Avoid dehydration and hypercalcaemia
Contraindications
The most important contraindication is if PD catheter access is not feasible.
Other major clinical contraindications include:5,9,22
- Inflammatory abdominal diseases including Crohn’s disease, ulcerative colitis, current clostridium difficile infection
- End-stage liver disease with ascites
Main anatomical contraindications include:5,9,22
Other contraindications include:5,9,22
- Non-functional peritoneal membrane
- All other health conditions (e.g. COPD) are relative contraindications
Anuria is not a contraindication.5 It is well recognised that preserving residual renal function is an important goal for PD, but it should not be the sole consideration while selecting the initial dialysis modality due to the lack of strong evidence.
Complications
PD catheter-related complications
Early complications (<30 days)10
- PD catheter obstruction: causes include constipation, catheter malposition/migration or catheter occlusions (due to kinking, thrombus, fibrin, omental wrapping, or adhesions)
- Cather mispositioning into the upper abdomen or omentum
- Bowel perforation (rare)
An obstructed catheter can be irrigated with saline or urokinase if caused by an intra-luminal obstruction. Otherwise, laparoscopic exploration and subsequent management may be required.10
Late complications (>30 days)10
- Infection of the exit site or tunnel, which may progress to bacterial peritonitis
- Cuff protrusion
- Outflow failure: likely due to constipation
- Peritoneal leak: dialysate may leak down the catheter tunnel into subcutaneous tissue
Membrane-related complications
A range of peritoneal membrane-associated complications can occur:
- PD-associated peritonitis: leading cause of PD discontinuation and needing to switch to HD.23
- Peritoneal fibrosis: associated with long-term PD and is another leading cause of PD discontinuation. It can cause membrane failure where there is insufficient dialysis despite a patent PD catheter and appropriate PD prescription.24
- Sclerosing encapsulating peritonitis (SEP): a potentially fatal complication of PD, where the progressively thickened peritoneal membrane encases the small bowel with adhesions and strictures, resulting in recurrent episodes of small bowel obstruction.25
PD-associated peritonitis
Peritonitis is a common and serious complication of PD, it is the direct contributing cause of death in >15% of patients.26 Most cases are secondary to contamination with pathogenic skin commensal bacteria during exchange or due to an exit-site or tunnel infection. It is typically caused by coagulase-negative staphylococcus, especially staphylococcus epidermidis.12,27
As per ISPD (International Society for Peritoneal Dialysis) guidelines, PD-associated peritonitis can be diagnosed when at least two of the following are present:27
- Clinical features consistent with peritonitis: abdominal pain and/or cloudy dialysis effluent
- Dialysis effluent white cell count > 100/µL: > 50% neutrophils, after a dwell time of at least two hours
- Positive dialysis effluent culture
Management of PD-associated peritonitis12,27
- Clinical examination, including the exit site, exit lumen and catheter tunnel
- Collect PD fluid for cell count, differential count (% of each type of leukocyte present), gram staining and culture
- Start intra-peritoneal (IP) antibiotics (first-generation cephalosporin for gram-positive coverage and third generation cephalosporin for gram-negative coverage) as soon as possible following collection of PD fluid and allow to dwell for at least six hours.
Metabolic complications
Cardiovascular disease is the leading cause of death in patients on PD.28,29
The development of overhydration after loss of residual renal function is thought to be the most important cardiovascular risk factor specific to PD.28,29
Furthermore, dialysate used in PD has a high glucose content, ranging from 25-75g of glucose per bag.21 Systemic glucose absorption causes hyperglycaemia, insulin resistance, and dyslipidaemia, creating a pro-atherosclerotic state, thus increasing the risk of cardiovascular diseases.
To minimise metabolic complications, the renal association guidelines recommend using glucose-sparing dialysate regimens where appropriate.19
Lastly, PD is also associated with hypokalaemia, which is also found to be associated with an increased risk of cardiovascular events.28
Fluid dwelling related-complications
The following complications are associated with the presence of fluid in the peritoneal cavity:12
- Hernias: caused by chronically raised intra-abdominal pressure from the indwelling dialysate
- Pain: may be associated with either inflow or drain phases of PD exchange
- Abdominal fullness: due to the presence of intraperitoneal fluid
Reviewer
Dr Roberta Callus
Consultant Nephrologist
Editor
Dr Jess Speller
References
- National Institute for Health and Care Excellence (NICE). Renal replacement therapy and conservative management (Clinical guideline [NG107]). NICE 2018. Published 2018 Oct. Available from: [LINK]
- Floege J, Johnson RJ, Feehally J. Comprehensive Clinical Nephrology, 4th edition. London: Elsevier Saunders 2010.
- Malhotra A. Acute Kidney Injury (AKI). Geriatric Trauma and Acute Care Surgery 2017 Jul;367-380
- Baker JB, Navarro Y, Sisroe TA, Everett C. Indications for Urgent and Emergent Hemodialysis. Annals of Vascular Surgery 2024 Jan;98:39-40.
- Chan CT, et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International 2019 Jul;96(1):37-47.
- Vadakedath S, Kandi Venkataramana. Dialysis: A Review of the Mechanisms Underlying Complications in the Management of Chronic Renal Failure. Cureus 2017 Aug;9(8):e1603.
- Murdeshwar HN, Anjum F. Hemodialysis. Treasure Island (FL): StatPearls Publishing 2024 Jan [Internet]. Updated: 2023 Apr. Available from: [LINK]
- Andreoli MCC, Totoli C. Peritoneal Dialysis. Revista da Associação Médica Brasileira 2020; 66(1):37-44.
- Sachdeva B, Zulfiqar H, Aeddula NR. Peritoneal Dialysis. Treasure Island (FL): StatPearls Publishing 2024 Jan [Internet]. Updated: 2023 Aug. Available from: [LINK]
- Peppelenbosch A, et al. Peritoneal dialysis catheter placement technique and complications. NDT Plus 2008 Oct;1(4):23-28.
- Mehrotra R, et al. The Current State of Peritoneal Dialysis. Journal of the American Society of Nephrology 2016 Nov;27(11):3238-3252.
- Teitelbaum I, Burkart J. Peritoneal dialysis. American Journal of Kidney Diseases 2003 Nov;42(5):1082-1096.
- NHS Dialysis. Last reviewed: 2021 Sep. Available from: [LINK]
- Peritoneal dialysis (PD). Kidney Care UK. Available from: [LINK]
- Rabindranath KS, et al. Continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis for end-stage renal disease. Cochrane Database of Systematic Reviews 2007 Apr;2007(2):CD006515
- Mehrotra R, et al. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney International 2009 Jul;76(1):97-107.
- Bieber SD, et al. Comparative Outcomes Between Continuous Ambulatory and Automated Peritoneal Dialysis: A Narrative Review. American Journal of Kidney Diseases 2014 Jun;63(6):1027-1037.
- Roberts G, et al. Current costs of dialysis modalities: A comprehensive analysis within the United Kingdom. Sage Journals 2022 Nov;42(6):578-584.
- Woodrow G, et al. Renal Association Clinical Practice Guideline on peritoneal dialysis in adults and children. BMC Nephrology 2017 Nov;18:333.
- Brown EA, et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Journal of the International Society for Peritoneal Dialysis 2020 May;40(3):244-253.
- Steddon, S. et al. Chapter 4 Dialysis. Oxford Handbook of Nephrology and Hypertension. Oxford: Oxford University Press, 2018.
- Al-Natour M, Thompson D. Peritoneal Dialysis. Seminars in Interventional Radiology 2016 Mar;33(1):3-5.
- Boissinot L, et al. Is Transition Between Peritoneal Dialysis and Haemodialysis Really a Gradual Process? Journal of the International Society for Peritoneal Dialysis 2013 Jul-Aug;33(4):391-397.
- Terri M, et al. Mechanisms of Peritoneal Fibrosis: Focus on Immune Cells-Peritoneal Stoma Interactions. Frontiers in Immunology 2021 Mar;12:607204.
- Machado NO. Sclerosing Encapsulating Peritonitis. Sultan Qaboos University Medical Journal 2016 May;16(2):142-151.
- Szeto CC, Li PKT. Peritoneal Dialysis-Associated Peritonitis Clinical Journal of the American Society of Nephrology 2019 Jul;14(7):1100-1105.
- Li PKT, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Sage Journals 2022 Mar;42(2).
- Mehrotra R. Chapter 32 Metabolic Complications of Peritoneal Dialysis. Handbook of Dialysis Therapy, 6th Elsevier 2023.
- Krediet RT, Balafa O. Cardiovascular risk in the peritoneal dialysis patient. Nature Reviews Nephrology 2010 Jun;6:451-460.




