- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE, Medicine, Surgery, Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Introduction
Type 2 diabetes mellitus (T2DM) is a common and complex metabolic disease characterised by insulin resistance and subsequent high blood glucose levels (hyperglycaemia).
Its complications are severe and affect multiple organ systems, causing cardiovascular disease (CVD), kidney disease, neuropathy and retinopathy.
Over 5 million people in the UK have diabetes, and 90% of these cases are due to T2DM.1,2 The NHS spends 10% of its annual budget on diabetes (over £10 billion), making it a major priority in terms of treatment and prevention.3
Aetiology
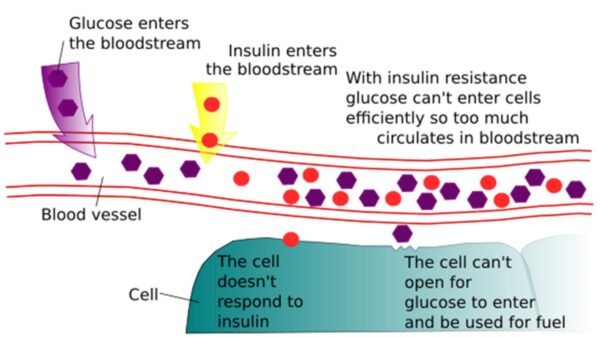
The pathophysiology of T2DM centres around insulin resistance, which is a complex process that has been simplified here.
Normally, plasma glucose levels are tightly regulated by the hormone insulin. Upon eating carbohydrate foods (containing sugar), plasma glucose levels rise. In response, the pancreas secretes insulin to bring glucose levels down by promoting uptake into organs such as the liver and skeletal muscle.
In T2DM, chronic high insulin levels (chronic hyperinsulinaemia) from excess dietary sugar intake (and other factors such as obesity) cause these organs to develop resistance to the effects of insulin, meaning that more insulin is needed to lower glucose to the same degree. This leads to chronic hyperglycaemia.4
Initially, insulin levels remain very high to try to “override” the resistance. However, as the condition progresses, the pancreas begins to “tire” and stops producing insulin. Insulin deficiency is the end-stage of T2DM – unlike in type 1 diabetes, where it is the main pathology (autoimmune destruction of insulin-producing of pancreatic beta-islet cells).4
Complications of diabetes result from glucose-related damage to blood vessels supplying vital organs. Hyperinsulinaemia also promotes weight gain (insulin is a “storage” hormone which prevents breakdown of adipose tissue) and hyperglycaemia increases the risk of infection/inflammation.4
Risk factors
Risk factors for developing T2DM include non-modifiable factors, modifiable factors and specific conditions.6
Non-modifiable risk factors
Non-modifiable risk factors include:
- Age: >40 years old, although now being seen more in younger people
- Sex: slightly more common in men than women
- Ethnicity: South Asian, Black African and Black-Caribbean groups have a higher risk
- Family history: especially having first-degree relatives with T2DM
Modifiable risk factors
Modifiable risk factors include:
- Obesity: encompasses increased body mass index (BMI)/waist circumference, sedentary lifestyle and consumption of processed carbohydrate/sugary foods.
- Alcohol excess: beer and cocktails are high in calories and sugar, and alcoholism causes liver damage, which reduces the hepatic uptake of glucose.
- Smoking: a potent CVD risk factor itself and also associated with insulin resistance.
- Poor sleep/stress: both promote insulin resistance and increase hyperglycaemia through chronically raised cortisol levels and poor dietary/exercise habits.
Medical conditions
Specific medical conditions which increase the risk of T2DM include:6,7,8
- Polycystic ovarian syndrome (PCOS): causes insulin resistance related to high androgen levels
- Gestational diabetes mellitus (GDM): diabetes in pregnancy usually resolves post-partum but has a high lifetime of developing T2DM
- Mental health conditions and related medications (antipsychotics)
- Steroid-induced diabetes: increases liver gluconeogenesis (production of glucose) and insulin resistance
Clinical features
History
The classic symptom triad of diabetes occurs due to insulin deficiency (“osmotic” symptoms), which occurs more in type 1 diabetes than type 2 diabetes:
- Polyuria (excess urinary volume): kidneys filter out more glucose into the urine, which, due to an osmotic effect, causes water to follow (osmotic diuresis)
- Polydipsia (excess thirst)
- Unintentional weight loss
Other common symptoms include:
- Tired all the time (TATT)
- Frequent/resistant infections, especially genitourinary infections such as urinary tract infection (UTI) & thrush due to glycosuria (glucose in urine)
- Poor-healing wounds
- Blurred vision (from diabetic eye complications such as retinopathy)
Many with T2DM are asymptomatic and are diagnosed incidentally on routine blood tests or during hospital admission after a complication (such as stroke or myocardial infarction (MI)).
Clinical examination
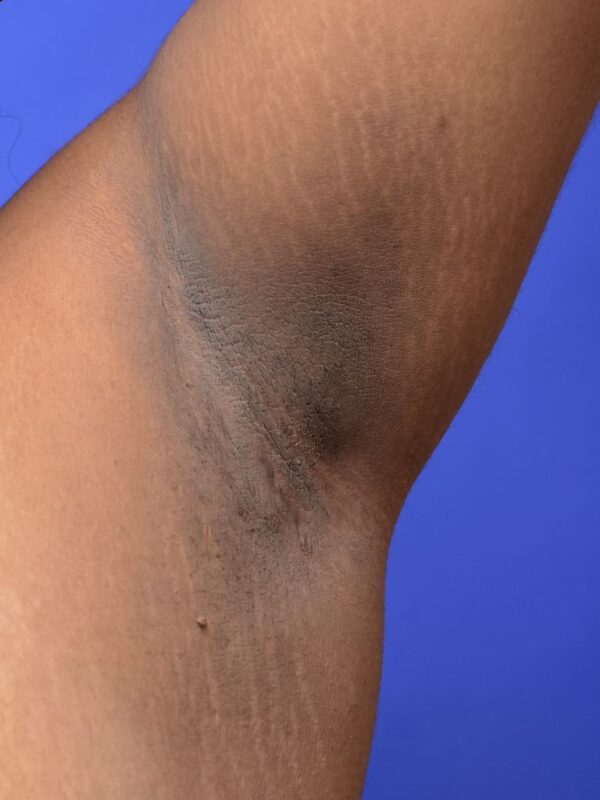
T2DM without complications may not have any physical signs. Raised BMI and waist circumference are common, while more specific signs of insulin resistance, such as acanthosis nigricans (darkened, thickened skin in folds/creases), are rare.
Clinical examination findings may be caused by complications of diabetes, including:
- Diabetic retinopathy on fundoscopy
- Evidence of diabetic neuropathy and peripheral vascular disease on foot examination
- Evidence of renal replacement therapy (e.g. arteriovenous fistula or renal transplant scar)
Differential diagnoses
The symptoms of T2DM overlap with many conditions.
Polyuria and/or polydipsia can also be caused by
- Diabetes insipidus (DI): anti-diuretic hormone (ADH) deficiency causing increased water loss from the kidneys
- Diuretics
- Psychogenic polydipsia
Tired all the time (TATT) has a wide differential, which includes conditions such as
- Anaemia
- Hypothyroidism
- Chronic kidney disease (CKD)
- Heart failure
- Malignancy
- Obstructive sleep apnoea (OSA)
- Depression/anxiety
Investigations
Bedside investigations
Bedside tests are not diagnostic but can be suggestive of the condition or complications.
Capillary blood glucose (“BM”)
Measured by a fingerprick test. BM stands for Boehringer Mannheim (the original pharmaceutical company involved).
Normal BM ranges (mmol/L):10
- Pre-meal = 4-5.9
- 2 hours post-meal = ≤7.8
Urine dipstick (urinalysis)
Urinary glucose (glycosuria) is common.
Urinary ketones (ketonuria) in an unwell patient can indicate diabetic ketoacidosis (DKA), an acute life-threatening complication of insulin deficiency (usually in type 1 diabetes).
Urinary protein (proteinuria) can indicate diabetic nephropathy.
Laboratory investigations
HbA1C
The primary blood test to diagnose and monitor T2DM is HbA1C (glycosylated haemoglobin).11
HbA1c is a measure of average blood glucose concentration (using glucose bound to haemoglobin/Hb) as a surrogate marker) in the preceding 3 months (average lifespan of a red blood cell/RBC). It cannot be used in circumstances where Hb is abnormal (e.g. haemoglobinopathies) or where RBCs are broken down early (e.g. haemolytic anaemia).
Alternative tests include the original fasting or random plasma venous glucose (PVG) test or serum fructosamine.11
Other blood tests
Other important blood tests include:11
- Urea & electrolytes (U&E): to assess renal function, which can be impaired by diabetic nephropathy and certain diabetic medications (e.g. metformin).
- Liver function tests (LFT): can indicate fatty liver disease, which is associated with T2DM and metabolic syndrome (also a useful baseline as some anti-diabetic medications can interfere with liver function)
- Cholesterol/lipids: dyslipidaemia commonly co-exists with T2DM and is another important CVD risk factor
Urine albumin-creatinine ratio (UACR)
Urine albumin-creatinine ratio (UACR) is an early morning urine sample which detects microalbuminuria (albumin in urine) and compares it with creatinine (a normal urinary waste product).
In diabetic nephropathy, glomerular basement membrane damage allows small proteins like albumin to leak abnormally through the filtration system into the urine – leading to a raised UACR. This can be an early indicator kidney damage related to diabetes.12
Diagnosis
T2DM is diagnosed with a HbA1C ≥48mmol/mol11
- If symptomatic then just 1 result is sufficient
- If asymptomatic then repeat HbA1C in 2 weeks, and if also ≥48mmol/mol, then the diagnosis is confirmed
Prediabetes (non-diabetic hyperglycaemia)
A condition where there is hyperglycaemia that does not reach the above threshold but has a significantly increased risk of progression to T2DM along with risks of complications – this is defined as HbA1C 42-47mmol/mol.13
Plasma venous glucose (PVG) was previously used for diagnosis, and the below criteria are useful to remember (repeat a second test if asymptomatic).
Table 1. Interpretation of plasma venous glucose.
| Test | Interpretation |
| Fasting PVG (mmol/L) |
|
| Random PVG (mmol/L) |
|
| Oral glucose tolerance test (OGTT) |
|
Management
Lifestyle
Modification of lifestyle plays a central role. Weight loss of >5% body weight (or more if obese) can put T2DM into remission or prevent the development of T2DM in >50% of those with pre-diabetes.15
Structured education on the following factors are important:
- Diet: improved glycaemic control can be achieved with diets low in sugar, especially with low carbohydrate and low ultra-processed food diets. These diets improve insulin resistance and promote weight loss. Low-calorie diets (800-1500kcal/day) can lead to significant weight loss and rapid reversal of T2DM, forming the basis for the NHS Type 2 Diabetes Path to Remission Programme.15,16
- Exercise: increased physical activity such as walking, high-intensity cardio and resistance training can improve insulin resistance. Aiming for ≥2.5 hours of physical activity a week is recommended. Muscle mass is particularly important for the uptake of plasma glucose (stored as glycogen).17
- Reducing alcohol consumption (<14 units/week), stopping smoking, and improving sleep and stress are also important changes.
Anti-diabetic drugs
It is common for newly diagnosed patients to undergo a period of lifestyle change. However, if HbA1C remains elevated or complications develop, then oral drug treatment is usually offered.
Metformin is the first-line drug, but beyond this, the next choices depend on individual patient circumstances and risk profiles. These are summarised below:18
Table 2. A summary of anti-diabetic drugs
| Drug | Mechanism | Side-effects/risks |
| Metformin | 1st line drug for T2DM in the biguanide family, which works to
|
|
| SGLT-2 inhibitors | e.g. Dapagliflozin/empagliflozin, which have become 2nd line due to cardio/reno-protective effects and works to
|
|
| Sulfonylureas | e.g. Gliclazide which is an older drug which works to
|
|
| DPP4 inhibitors | e.g. Sitagliptin/Linagliptin which increase the “incretin” effect
|
|
| GLP-1 analogues | e.g. Liraglutide, Semaglutide which are newer subcutaneous (SC) injections which
|
|
| Other less-used options |
|
|
HbA1C targets and thresholds
HbA1C targets should be individualised (e.g. a younger patient with signs of early complications or diagnosis <10 years ago may have stricter targets, whereas an older patient at risk of falls and polypharmacy may have relaxed targets).
Table 3. A general guide to HbA1c targets.
| Situation | HbA1C (mmol/mol) |
| On lifestyle or metformin alone | ≤48 |
| On >1 drug or any drug causing hypoglycaemia | ≤53 |
| Intervention threshold for starting a 2nd drug (if on metformin) or a 3rd drug (triple therapy) if already on 2 drugs | ≥58 |
Insulin
If the above treatment fails (i.e. already on “triple therapy” or established complications) or rapid control is required (e.g. due to very high HbA1C or osmotic symptoms), then subcutaneous insulin injections may be needed.20
Insulin is the mainstay treatment for type 1 diabetes, which is a disease of insulin deficiency from the start, whereas in T2DM, insulin deficiency occurs much later.
Insulin comes in many forms and is often started as a twice-daily intermediate-acting preparation (unlike the traditional “basal-bolus” regimen used in type 1 diabetes), alongside metformin.
The main risks of insulin are hypoglycaemia and weight gain. Lipodystrophy from recurrent subcutaneous injections can also occur.
BM monitoring is important; however, new technologies such as continuous glucose monitoring (CGM) and insulin pumps have become more widely available.
Cardiovascular risk reduction
It is important to optimise other CVD risk factors (such as hypertension, hyperlipidaemia, and CKD) to prevent MI and stroke, and these aspects link closely with one another.
CVD risk calculators (such as QRISK3, which calculates the 10-year risk of having a cardiovascular event) are used to assist with this.
Hypertension, microalbuminuria and CKD19
Target BP in T2DM is <140/90mmHg in clinic (<135/85mmHg for home/ambulatory monitoring)
The first-line drug treatment is ACE inhibitors (ACE) (e.g. ramipril) or angiotensin receptor blockers (ARB) (e.g. losartan) regardless of age
If there is CKD (with microalbuminuria), then tighter control may be needed.
Table 4. Management of microalbuminuria.
| UACR (mg/g) | Management |
| >3 | Should be on ACE or ARB regardless of BP (even if normotensive) |
|
>30 |
If on the maximum dose of ACE/ARB add in dapagliflozin (SGLT-2) |
| >70 | Stricter BP target <130/80mmHg in clinic (or <125/75mmHg at home or ambulatory monitoring) and refer to renal specialist |
Cholesterol and statins19
Any patient with T2DM with QRISK >10% should be offered a statin (atorvastatin 20mg) as primary prevention of CVD.
If an MI/stroke occurs, then a high-intensity statin (80mg atorvastatin) should be started as secondary prevention.
Complications
The complications of T2DM occur primarily due to vascular damage caused by hyperglycaemia and can be split into macrovascular and microvascular.21
Macrovascular (large vessel) complications include:
- Myocardial infarction (MI) and associated ischaemic heart disease (IHD)
- Stroke or transient ischaemic attack (TIA) and associated cerebrovascular disease
- Peripheral vascular disease (PVD) causing intermittent claudication and critical limb ischaemia, with an associated risk of infection, ulceration, necrosis and amputation (“Diabetic foot”)
Microvascular (small vessel) complications include:
- Diabetic nephropathy causing CKD, initially starting with microalbuminuria, progressing to end-stage renal disease
- Diabetic retinopathy causing visual loss and blindness – requires annual screening
- Diabetic neuropathy causing peripheral nerve damage and with PVD increases risk of infection, trauma, ulceration and amputation (“Diabetic foot”)
Other complications related to diabetes include Charcot’s foot/arthropathy (red hot swollen foot with osteolysis and deformity), frozen shoulder, carpal tunnel syndrome and De Quervain’s tenosynovitis.
Diabetes annual review checklist22
- HbA1C and individualised target (as well as compliance with medications)
- Lifestyle advice and education
- Bedside parameters – BMI, BP, urine dip for protein (UACR if positive)
- Other lab tests – U&E, lipids & calculation of QRISK
- Diabetic foot check
- Inspection for footwear, ulcers, infection, gangrene etc.
- Palpation of foot pulses and capillary refill time
- Neuropathy testing of sensation (using a 10g monofilament)
- Referral to orthotics, podiatrists and specialists may be needed
- Ensure annual diabetic retinopathy screening
Implications for driving
The DVLA has specific driving regulations for diabetes, which depend on whether the patient holds a Group 1 (normal car) or Group 2 (Heavy Goods Vehicle/HGV) license.23
If T2DM is managed with lifestyle alone or medications that do not cause hypoglycaemia, the DVLA does not need to be informed. However, for those on hypoglycaemic drugs (gliclazide, insulin), the DVLA should be informed, and specific criteria will be assessed to decide whether they are fit to drive.
Key points
- T2DM is a complex multi-organ disease caused by hyperglycaemia related to insulin resistance, with obesity and family history playing an important role
- HbA1C is the main test for diagnosis, and other important tests include measurement of BP, BMI, UACR, U&E and lipids to assess risk of cardiovascular disease
- Management involves lifestyle change, especially weight loss, and anti-diabetic drugs such as metformin, dapaglifozin and gliclazide. Insulin is a last resort.
- Complications include CVD, nephropathy, retinopathy and neuropathy and should be assessed annually. There may be implications for driving.
Reviewer
Professor Parth Narendran
Consultant Diabetologist
Editor
Dr Chris Jefferies
References
- Diabetes UK. Number of people living with diabetes in the UK tops 5 million for the first time. Available from: [LINK]
- Diabetes UK. Us, diabetes and a lot of facts and stats. 2019. Available from: [LINK]
- NHS England. NHS Prevention Programme cuts chances of Type 2 diabetes for thousands. 2022. Available from: [LINK]
- De Fronzo et al. Type 2 diabetes mellitus. Nature Reviews (2015). Available from: [LINK]
- Image: Ozhank. License: [CC0 1.0]
- Diabetes UK. Diabetes risk factors. Accessed 2024. Available from: [LINK]
- Vounzoulaki et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ (2020). Available from: [LINK]
- Diabetes UK. Steroid-induced diabetes. Accessed 2024. Available from: [LINK]
- Image: Mark Brady; Prashanth Rawla, License: [CC BY-SA 4.0].
- Diabetes.co.uk. NICE recommended target blood glucose level ranges (Factsheet). Accessed 2024. Available from: [LINK]
- NICE CKS. Diabetes – type 2. When should I suspect type 2 diabetes in an adult? Last revised in November 2023. Available from: [LINK]
- GP Notebook. NICE guidance – diabetic renal disease in type II diabetes. Last reviewed 24 Oct 2023. Available from: [LINK]
- Diabetes UK. Prediabetes. Accessed 2024. Available from: [LINK]
- Diabetes.co.uk. Oral Glucose Tolerance Test. Updated in 2023. Available from: [LINK]
- NHS England. NHS Type 2 Diabetes Path to Remission Programme. Accessed 2024. Available from: [LINK]
- Forouhi et al. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ (2018). Available from: [LINK]
- Colberg et al. Exercise and Type 2 Diabetes. Diabetes Care (2010). Available from: [LINK]
- NICE (NG28). Visual summary on choosing medicines for type 2 diabetes. 2022. Available from: [LINK]
- NICE CKS. Diabetes – type 2: Scenario: Management – adults. Updated November 2023. Available from: [LINK]
- NICE CKS. Scenario: Insulin therapy – type 2 diabetes. Updated January 2021. Available from: [LINK]
- NICE CKS. Diabetes – type 2: What are the complications? Updated November 2023. Available from: [LINK]
- Diabetes UK. Diabetes care to expect. Accessed 2024. Available from: [LINK]
- DVLA. Diabetes and driving. Accessed 2024. Available from: [LINK]




